Merck & Co. (NYSE:MRK) of Kenilworth, NJ, is one of the world’s largest pharmaceutical companies and it markets cholesterol inhibitors like Zetia as well as diabetes medications such as Januvia. In mid-January, Merck announced that it would be acquiring Britain-based drug discovery firm IOmet, a company which holds some intriguing properties in cancer immunotherapy. The move, which makes IOmet a wholly owned subsidy of Merck, adds a number of IDO, TDO and dual IDO/TDO inhibitors to Merck’s pre-clinical pipeline. These inhibitors are designed to target enzymes which are overexpressed in cancer and lead to tryptophan depletion in patients. The deal is also expected to benefit the University of Dundee in Scotland, where researchers worked on advances contributing to IOmet’s immuno-oncology products.
On Wednesday, February 3rd, Merck released its latest earnings report for the fourth quarter of 2015 and the company beat its estimated earnings per share (EPS) with 93 cents EPS for the quarter, two cents better than the 91 cents EPS analysts predicted. However, Merck’s worldwide sales were down during the quarter by about 3 percent to $10.2 billion with drooping sales figures for type 2 diabetes treatment Januvia, rheumatoid arthritis medication Remicade and HIV treatment Isentress. The company did see more than 100 percent growth in sales for its antibiotic product Cubicin and the tumor immunotherapy drug Keytruda compared to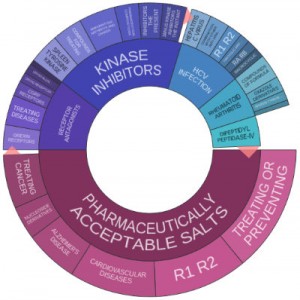 their performance in the fourth quarter of 2014.
their performance in the fourth quarter of 2014.
Merck doesn’t appear in IFI Claims Top 50 U.S. Patent Assignees for 2015 but the company actively pursues protection for its intellectual properties from the U.S. Patent and Trademark Office. In 2014, Merck placed 125th overall with 293 patents earned during that year, an increase of 9.3 percent over the number of U.S. patents earned by the pharmaceutical firm in 2013. The patent portfolio analysis tools available through Innography show us that the company earned 224 U.S. patents during 2015, a sizable dropoff from its 2014 results. The text cluster shows us that, unsurprisingly, a great deal of Merck’s 2015 innovations focused upon pharmaceutically acceptable salts, although we’re seeing significant activity in receptor agonists, hepatitis C virus (HCV) infection, kinase inhibitors and rheumatoid arthritis as well.
Merck’s Recently Issued Patents: From Hepatitis C Inhibitors to Dengue Virus Treatments
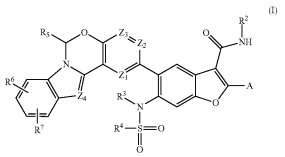 We noticed that a couple of patents issued in recent weeks to Merck focus directly on treating patients suffering from HCV, including the pharmaceutical treatment outlined in U.S. Patent No. 9242998, titled Tetracyclic Heterocycle Compounds and Methods of Use Thereof for the Treatment of Hepatitis C. It protects compounds of a formula useful as HCV non-structural protein 5B (NS5B) polymerase inhibitors. This innovation is based upon research which has found that antagonists of NS5B activity in a patient with HCV serves as an effective inhibitor of HCV replication and can be combined with other HCV medications. Inhibitory compounds that regulate the activity of this virus in a patient are also disclosed within U.S. Patent No. 9242917, entitled Crystal Forms of a HCV Protease Inhibitor. It claims a compound including multiple hydrates and crystal sodium salt that can be administered to a patient as a treatment for HCV. The treatment includes macrolactam compounds which have shown the ability to inhibit HCV activity in vivo and in vitro and also inhibits NS3 enzymatic activity to check the spread of HCV.
We noticed that a couple of patents issued in recent weeks to Merck focus directly on treating patients suffering from HCV, including the pharmaceutical treatment outlined in U.S. Patent No. 9242998, titled Tetracyclic Heterocycle Compounds and Methods of Use Thereof for the Treatment of Hepatitis C. It protects compounds of a formula useful as HCV non-structural protein 5B (NS5B) polymerase inhibitors. This innovation is based upon research which has found that antagonists of NS5B activity in a patient with HCV serves as an effective inhibitor of HCV replication and can be combined with other HCV medications. Inhibitory compounds that regulate the activity of this virus in a patient are also disclosed within U.S. Patent No. 9242917, entitled Crystal Forms of a HCV Protease Inhibitor. It claims a compound including multiple hydrates and crystal sodium salt that can be administered to a patient as a treatment for HCV. The treatment includes macrolactam compounds which have shown the ability to inhibit HCV activity in vivo and in vitro and also inhibits NS3 enzymatic activity to check the spread of HCV.
Cancer treatments are also at the center of a couple of Merck patents that we explored during our recent survey, such as is reflected in U.S. Patent No. 9229008, titled IL-8 Level as a Determinant of Responsivity of a Cancer to Treatment. It claims a method of determining whether a patient has a malignant tumor or blood cancer which is responsive to an agent by determining an IL-8 level in the subject’s blood or serum, administering the agent to the subject and then determining an IL-8 level in the subject’s blood or serum again; the cancer is determined to be responsive to the agent if the IL-8 level decreases. Monitoring the pro-inflammatory cytokine IL-8 in this way can determine the effect that an extracellular signal regulated kinase (ERK) inhibitor will have on a patient’s cancer. That technology would be used to judge the effectiveness of innovative treatments such as is outlined within U.S. Patent No. 9226922, titled Compounds that are ERK Inhibitors. It discloses a compound having a pyrazolopyridine base structure and pharmaceutically acceptable salts thereof. This innovation introduces the use of small-molecules to serve as an ERK inhibitor against a broad spectrum of cancers, including melanoma, lung cancer, breast cancer, pancreatic cancer and ovarian cancer.
Merck is also focused on research and development in treating insect-borne diseases, as is evidenced by the issue of U.S. Patent No. 9198964, which is titled Recombinant Subunit Dengue Virus Vaccine. It protects an immunogenic composition comprising an effective amount of purified dengue virus envelope protein monomers of various serotypes and an effective amount of adjuvant; the envelope protein monomers are secretable into a growth medium when expressed recombinantly in a host cell to induce the production of neutralizing antibodies in human subjects. The treatment is designed to provide balance and effective immune responses to different strains of the dengue virus while providing an acceptable safety profile.
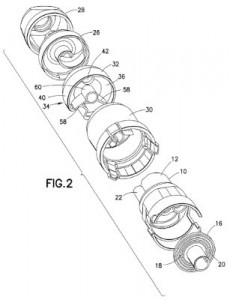 The administration of drug products, especially dry powder treatments, is improved by the invention discussed within U.S. Patent No. 9174012, entitled Drug Products, Dry Powder Inhalers and Polyflux Collider Arrangements. It claims a polyflux collider arrangement for de-agglomerating dry powder in a powder dispenser during inhalation of a dose of dry powder and having spaced-apart first and second inlet openings, a reference plane passing through the center of those openings and a polyflux collider with a body at least partially encompassing a volume and having a single exit opening through which a collective stream of dry powder passes during dose administration. This inhaler components seeks to improve the efficacy of dry powder inhalers by delivering a higher fine particle fraction and a fine particle dose.
The administration of drug products, especially dry powder treatments, is improved by the invention discussed within U.S. Patent No. 9174012, entitled Drug Products, Dry Powder Inhalers and Polyflux Collider Arrangements. It claims a polyflux collider arrangement for de-agglomerating dry powder in a powder dispenser during inhalation of a dose of dry powder and having spaced-apart first and second inlet openings, a reference plane passing through the center of those openings and a polyflux collider with a body at least partially encompassing a volume and having a single exit opening through which a collective stream of dry powder passes during dose administration. This inhaler components seeks to improve the efficacy of dry powder inhalers by delivering a higher fine particle fraction and a fine particle dose.
Patent Applications of Note: Medication Mixing Syringes and Novel Diabetes Treatments
A couple of patent applications filed in recent months by Merck discuss treatments targeting the medical condition diabetes, such as is featured within U.S. Patent Application No. 20150359793, titled Beta-Amino Heterocyclic Dipeptidyl Peptidase Inhibitors for the Treatment or Prevention of Diabetes. It would protect a pharmaceutical composition including a first formula serving as an inhibitor of the dipeptidyl peptidase-IV (DP-IV) enzyme, insulin and a pharmaceutically acceptable carrier. This pharmaceutical innovation serves as a treatment for diabetes, particularly Type 2 diabetes, by inhibiting DP-IV which, in turn, increases the level of serum insulin in a patient. Diabetes and other lipid disorders are also targeted by the inventive treatment at the center of U.S. Patent Application No. 20160002255, filed under the title Antidiabetic Bicyclic Compounds. It claims a compound and pharmaceutically acceptable salts thereof which serve as agonists of G-protein coupled receptor 40 (GPR40). The inhibition of GPR40 may be useful in the treatment of Type 2 diabetes and other conditions affected by GPR40 activity, including hypertension, hyperglycemia, obesity and atherosclerosis.
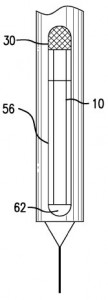 We were intrigued by a couple of patent application filings related to medical devices developed by Merck, including the medicine monitoring technology discussed within U.S. Patent Application No. 20150343157, filed under the title Medication Injector with Near-Empty Alert. It discloses a medication injector for injecting a medication and having a housing with a longitudinal axis and an internal viewing portion for viewing inside the housing, a piston rod in the housing oriented in the direction of the longitudinal axis and an alert indicator carried on the piston rod for signalling that the injector is reaching the end of its medication supply; the alert indicator moves between a hidden and a visible position based on the advancement of the piston rod. This method of informing injector pen users that a medication is running low has a much easier configuration than “active-stop” injector pens, which have numerous parts and are expensive to manufacture. A syringe which is capable of storing medication chemicals and properly mixing them just prior to administration to a patient is outlined within
We were intrigued by a couple of patent application filings related to medical devices developed by Merck, including the medicine monitoring technology discussed within U.S. Patent Application No. 20150343157, filed under the title Medication Injector with Near-Empty Alert. It discloses a medication injector for injecting a medication and having a housing with a longitudinal axis and an internal viewing portion for viewing inside the housing, a piston rod in the housing oriented in the direction of the longitudinal axis and an alert indicator carried on the piston rod for signalling that the injector is reaching the end of its medication supply; the alert indicator moves between a hidden and a visible position based on the advancement of the piston rod. This method of informing injector pen users that a medication is running low has a much easier configuration than “active-stop” injector pens, which have numerous parts and are expensive to manufacture. A syringe which is capable of storing medication chemicals and properly mixing them just prior to administration to a patient is outlined within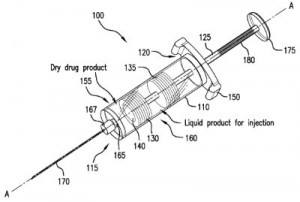 U.S. Patent Application No. 20150343153, entitled Dual Chamber Mixing Syringes and Methods of Using Same. This patent application would protect a dual-chamber mixing syringe having a hollow body with inner walls, a distal discharge end, a proximal piston rod receiving end, a piston rod extending into the hollow body through an opening in the piston rod receiving end, an axially-bored first piston, a second piston in actuating engagement with the piston rod such that the second piston moves axially toward the discharge end when the piston rod is depressed and a valve for reversibly sealing the axial bore in the first piston. This syringe is capable of delivering two-component drugs which are formed just prior to patient administration.
U.S. Patent Application No. 20150343153, entitled Dual Chamber Mixing Syringes and Methods of Using Same. This patent application would protect a dual-chamber mixing syringe having a hollow body with inner walls, a distal discharge end, a proximal piston rod receiving end, a piston rod extending into the hollow body through an opening in the piston rod receiving end, an axially-bored first piston, a second piston in actuating engagement with the piston rod such that the second piston moves axially toward the discharge end when the piston rod is depressed and a valve for reversibly sealing the axial bore in the first piston. This syringe is capable of delivering two-component drugs which are formed just prior to patient administration.
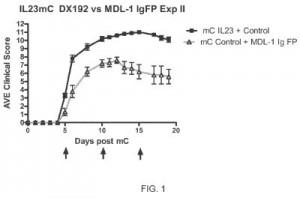 Pharmaceutical compositions aimed at suppressing unwanted immune responses during a course of treatment are detailed within U.S. Patent Application No. 20150368344, filed under the title MDL-1 Ligand. It would protect a method of modulating an interaction between a lectin-like molecule expressed on myeloid cells and a lectin which binds to a receptor expressed by a T cell; the method involves providing the compound that is capable of binding to a lectin-like molecule at a binding site of the lectin and presenting that compound to the lectin-like molecule and the lectin to modulate the interaction between the first and second lectin. The control of interactions involving MDL-1 interaction can aid patients by controlling induction of an immune pathway that would otherwise lead to chronic inflammation or improper clearance of infectious microbes. An inhibitory agent that has shown effectiveness against various conditions, including asthma and cancer, would be protected by U.S. Patent Application No. 20150353552, entitled Purine Inhibitors of Human Phosphatidylinositol 3-Kinase Delta. It claims a compound with a formula serving as an inhibitor of phosphatidylinositol 3-kinase (PI3K) delta useful in the treatment of PI3K-delta-mediated diseases. This treatment provides inhibition of PI3K-delta activity to prevent cancer cell growth and proliferation, as well as other autoimmune and inflammatory diseases, without disturbing other normal PI3K isoform activity.
Pharmaceutical compositions aimed at suppressing unwanted immune responses during a course of treatment are detailed within U.S. Patent Application No. 20150368344, filed under the title MDL-1 Ligand. It would protect a method of modulating an interaction between a lectin-like molecule expressed on myeloid cells and a lectin which binds to a receptor expressed by a T cell; the method involves providing the compound that is capable of binding to a lectin-like molecule at a binding site of the lectin and presenting that compound to the lectin-like molecule and the lectin to modulate the interaction between the first and second lectin. The control of interactions involving MDL-1 interaction can aid patients by controlling induction of an immune pathway that would otherwise lead to chronic inflammation or improper clearance of infectious microbes. An inhibitory agent that has shown effectiveness against various conditions, including asthma and cancer, would be protected by U.S. Patent Application No. 20150353552, entitled Purine Inhibitors of Human Phosphatidylinositol 3-Kinase Delta. It claims a compound with a formula serving as an inhibitor of phosphatidylinositol 3-kinase (PI3K) delta useful in the treatment of PI3K-delta-mediated diseases. This treatment provides inhibition of PI3K-delta activity to prevent cancer cell growth and proliferation, as well as other autoimmune and inflammatory diseases, without disturbing other normal PI3K isoform activity.
Finally, we were interested in an inventive pharmaceutical treatment designed to wipe out bacteria which has grown resistant to other drugs, which is laid out within U.S. Patent Application No. 20150337013, titled Novel Antibacterial Agents for the Treatment of Gram Positive Infections. It claims a method of preparing a compound and pharmaceutically acceptable salts thereof by providing a tert-butyloxycarbonyl-protected compound and removing the tert-butyloxycarbonyl protecting group of the compound. This invention addresses a need for novel antibiotics to address pathogens that have been repeatedly exposed to common antibiotics and have developed a resistance, especially in the case of Clostridium difficile which has become a growing concern for its ability to cause nosocomial infectious diarrhea.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)


![[[Advertisement]]](https://ipwatchdog.com/wp-content/uploads/2023/01/2021-Patent-Practice-on-Demand-1.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
No comments yet.