Abbott Laboratories (NYSE:ABT) of Chicago, IL, is poised to surge ahead in the global point-of-care medical diagnostics industry by acquiring Alere Inc. (NYSE:ALR) of Waltham, MA, in a $5.8 billion deal which values Alere at $56 per share. According a recent statement made by Abbott CEO Miles White to investors, the move will push the company’s annual diagnostic sales up to $7 billion. The acquisition is simply the latest major move in the medical device industry, a sector which saw more than 1,000 deals pending or completed last year for a net worth of $58.9 billion, according to statistics published by Bloomberg Business.
Throughout 2015, Abbott took quite a hit in value with its shares down 15 percent from the start of last year through December. Recently reported fourth quarter earnings showed earnings per share remain stagnant year-over-year at 62 cents per share and the company’s $5.2 billion in quarterly sales were also down 3.1 percent from 2014’s fourth quarter. Before the recent Alere news, financial analysts expected 2016 to further hurt Abbott’s fortunes, due in large part to the company’s exposure in emerging markets where exchange rates are growing less favorable. Abbott is also making its first foray into U.S. retail markets in 15 years when it begins sales of its Curate line of health snacks, which will include chocolate-quinoa-hemp seed and mission figs-balsamic vinegar snack bars.
On the other side, Alere share prices rose 43 percent in the week immediately following the news that Abbott’s purchase offer had been accepted. Last November, Alere had divested itself of a business group providing products for diagnostic, research, defense, healthcare and food industries, which it sold for $115 million to British-based private equity group Exponent Private Equity. Also in November, Alere received prequalification from the World Health Organization for the world’s first dual point-of-care test for both syphilis and HIV, allowing the test to be deployed in national screening programs in needy countries across the world.
Given the news of Abbott’s acquisition of Alere we thought we would take a look at the recent patent activity for both Abbott and Alere, which follows.
Abbott’s Patent Portfolio: Plenty of Medical Stent Innovations and Biodegradable Implants
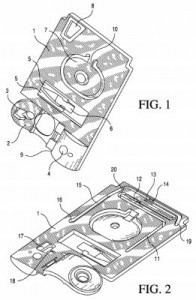
 Stents for blood vessels and vascular treatments were at the center of a series of patents we found in our survey of Abbott’s IP portfolio, such as can be seen in U.S. Patent No. 9253992, titled Cover Sleeve and Apparatus for Loading Material into a Stent Strut. It protects and apparatus for loading material into a stent strut and having an elastic cover sleeve forming a tube with an inner diameter and moving from an enlarged orientation to a reduced orientation in response to pressure differences within and outside of the tube, and then a stent support carrying a stent into the tube and movable into and out of the elastic cover sleeve. This innovation was sought to address challenges in filling a hollow stent with therapeutic agents for use in the treatment against atherosclerosis. The treating of medical conditions with agents which are deposited onto a medical stent is also the focus of U.S. Patent No. 9220759, entitled Treatment of Diabetic Patients with a Drug Eluting Stent and Adjunctive Therapy. It claims a method of treating, preventing or ameliorating a vascular disease in a diabetic patient by implanting a stent comprising a first drug in a vascular region of a diabetic patient and then administering a second drug to the diabetic patient via transdermal administration; the first drug may include anti-inflammatory, antiproliferative, antimitotic or antibiotic compositions, whereas the second drug reduces platelet activation, reduces thrombin activity or reduces tissue factor activity. This innovation is designed to reduce the formation of blood clots which can form long after stents have been implanted within a patient.
Stents for blood vessels and vascular treatments were at the center of a series of patents we found in our survey of Abbott’s IP portfolio, such as can be seen in U.S. Patent No. 9253992, titled Cover Sleeve and Apparatus for Loading Material into a Stent Strut. It protects and apparatus for loading material into a stent strut and having an elastic cover sleeve forming a tube with an inner diameter and moving from an enlarged orientation to a reduced orientation in response to pressure differences within and outside of the tube, and then a stent support carrying a stent into the tube and movable into and out of the elastic cover sleeve. This innovation was sought to address challenges in filling a hollow stent with therapeutic agents for use in the treatment against atherosclerosis. The treating of medical conditions with agents which are deposited onto a medical stent is also the focus of U.S. Patent No. 9220759, entitled Treatment of Diabetic Patients with a Drug Eluting Stent and Adjunctive Therapy. It claims a method of treating, preventing or ameliorating a vascular disease in a diabetic patient by implanting a stent comprising a first drug in a vascular region of a diabetic patient and then administering a second drug to the diabetic patient via transdermal administration; the first drug may include anti-inflammatory, antiproliferative, antimitotic or antibiotic compositions, whereas the second drug reduces platelet activation, reduces thrombin activity or reduces tissue factor activity. This innovation is designed to reduce the formation of blood clots which can form long after stents have been implanted within a patient.
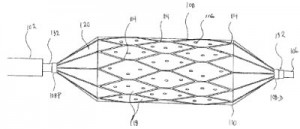
 Abbott has been involved in its own point-of-care research and development, as is reflected by U.S. Patent No. 9199234, entitled TSH Antibodies for Point-of-Care Immunoassay Formats. It discloses a device for performing an immunoassay for thyroid stimulating hormones (TSH) in a fluid sample, the device having an immunosensor in a conduit, the immunosensor having an antibody capture specific for TSH and the conduit having a dry reagent coating containing a signal antibody that dissolves into the fluid sample. This diagnostic point-of-care devices seeks to reduce the need for a wash step, which increases such a device’s cost as well as its environmental impact. Coating agents which improve the use of medical devices are also at the center of Abbott’s U.S. Patent No. 9220812, entitled Surface Interactions to Improve Retention of Medical Devices. The patent protects a method for improving the retention between surfaces of coatings by providing a plurality of coated medical devices each having a surface, where one coating surface has a host molecule and another coating surface has guest atoms or molecules, and then placing the surface containing a host molecule in proximity to the surface containing guest atoms or molecules such that a plurality of host-guest interactions are produced between the surfaces for improving retention between the devices. This innovation, also directed towards medical stents, improves a patient’s retention of a stent without trading off any characteristics in coating integrity, drug potency or additional deployment steps.
Abbott has been involved in its own point-of-care research and development, as is reflected by U.S. Patent No. 9199234, entitled TSH Antibodies for Point-of-Care Immunoassay Formats. It discloses a device for performing an immunoassay for thyroid stimulating hormones (TSH) in a fluid sample, the device having an immunosensor in a conduit, the immunosensor having an antibody capture specific for TSH and the conduit having a dry reagent coating containing a signal antibody that dissolves into the fluid sample. This diagnostic point-of-care devices seeks to reduce the need for a wash step, which increases such a device’s cost as well as its environmental impact. Coating agents which improve the use of medical devices are also at the center of Abbott’s U.S. Patent No. 9220812, entitled Surface Interactions to Improve Retention of Medical Devices. The patent protects a method for improving the retention between surfaces of coatings by providing a plurality of coated medical devices each having a surface, where one coating surface has a host molecule and another coating surface has guest atoms or molecules, and then placing the surface containing a host molecule in proximity to the surface containing guest atoms or molecules such that a plurality of host-guest interactions are produced between the surfaces for improving retention between the devices. This innovation, also directed towards medical stents, improves a patient’s retention of a stent without trading off any characteristics in coating integrity, drug potency or additional deployment steps.
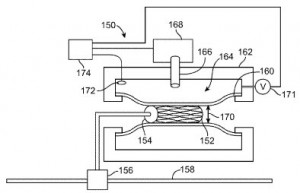
 Innovations involving drug delivery through a catheter are discussed within U.S. Patent No. 9216271, which is titled Local Delivery with a Balloon Covered by a Cage. This patent protects a method to deliver a liquid agent to a vascular lumen by delivering, to a treatment site, an assembly with an inflatable balloon substantially covered by an expandable stent-like structure with a plurality of struts and having a plurality of components isolated from blood flow, deploying the expandable stent-like structure within the vascular lumen against the lumen wall such that the plurality of components are isolated between the struts, the inflatable balloon’s outer surface and the vascular lumen’s lumen wall, inflating the inflatable balloon to the fully deployed state such that the balloon resides substantially inside the expandable stent-like structure and then delivering a liquid agent into the isolated compartments at the treatment site. This innovation is intended to reduce drug washout at a treatment site, which can cause harmful overexposure to a drug while reducing the effectiveness of the medical treatment device.
Innovations involving drug delivery through a catheter are discussed within U.S. Patent No. 9216271, which is titled Local Delivery with a Balloon Covered by a Cage. This patent protects a method to deliver a liquid agent to a vascular lumen by delivering, to a treatment site, an assembly with an inflatable balloon substantially covered by an expandable stent-like structure with a plurality of struts and having a plurality of components isolated from blood flow, deploying the expandable stent-like structure within the vascular lumen against the lumen wall such that the plurality of components are isolated between the struts, the inflatable balloon’s outer surface and the vascular lumen’s lumen wall, inflating the inflatable balloon to the fully deployed state such that the balloon resides substantially inside the expandable stent-like structure and then delivering a liquid agent into the isolated compartments at the treatment site. This innovation is intended to reduce drug washout at a treatment site, which can cause harmful overexposure to a drug while reducing the effectiveness of the medical treatment device.
Abbott’s R&D arm is also invested in improving cancer treatments, as readers can see by the issue of U.S. Patent No. 9186329, which is titled Method of Treating Malignant Solid Tumors. It discloses a method of delivering a drug to a malignant solid tumor by delivering a plurality of microparticles comprising a drug capable of inhibiting and/or the upregulation of mTOR through an artery to a point at or near the tumor, where the microparticles have a mean particle size small enough to pass through a metarteriole but large enough to be trapped by a capillary having a diameter ranging from 4 microns to 15 microns, and then subsequently administering a chemotherapeutic agent or radiation therapy. This treatment is designed to reduce the upregulation of the Akt/mTOR pathway in order to reduce resistance to chemotherapeutic agents administed to a patient.
Implantable stents which have characteristics for safe biodegradation within the body of a patient are the focus of U.S. Patent No. 9186440, titled Polyester Implantable Medical Device with Controlled in Vivo Biodegradability. This patent claims a stent having a blend comprising or formed from a high molecular weight polyester and a medium molecular weight polyester which are different, the medium molecular weight polyester having at least a portion substituted with an acidic moiety. The resulting invention is a polymeric implantable medical device with improved strength and toughness characteristics while also being fully biodegradable over time, reducing the chances of developing late stent thrombosis.
Alere’s Patent Portfolio: More Diagnostic Device Innovations and Electronic Systems for Test Result Management
Alere brings to Abbott a sizable lineup of medical diagnostics, point-of-care and testing devices, this last area being bolstered by recently issued patents such as U.S. Patent No. 9052311, which is titled Assay Device. It protects an assay device to determine the presence of at least one analyte of interest in a liquid sample and including a test strip onto which is deposited a first directly-labeled analyte-specific binding reagent for generating a test signal and a second directly-labeled specific binding reagent generating a second signal which indicates that a test has been successfully conducted and that a sufficient amount of time has elapsed following contact of the assay device with the liquid sample so that a test signal has been properly generated. This innovation, which can be applied to pregnancy tests and drug screening devices alike, modifies a control signal indicating that a test was successful so that the signal occurs after a predetermined length of time. Diagnostic testing devices meant for organizational compliance with workplace safety 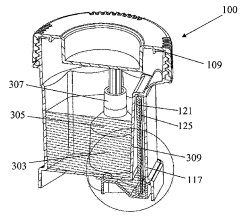 laws are detailed within U.S. Patent No. 9011770, entitled Device for Detecting Analytes in Fluid Samples. It protects a device for detecting the presence of an analyte in a liquid sample and having an opening for introducing the liquid sample into a first chamber for collecting the sample, a second chamber connected to the first chamber through a passageway and containing a test element, a third chamber connected to the second through a passageway and containing a plunger with a push bar that separates the third chamber into first and second zones, and then a lid for closing the opening and contacting the push bar to move the plunger, causing a volume of air to be drawn from the second chamber into the second zone of the third chamber, which itself causes a volume of the liquid sample to be drawn from a first chamber through the first passageway into the second chamber to contact the test element. The resulting invention provides a device through which the accurate testing of a subject can be easily performed by someone other than a trained healthcare professional.
laws are detailed within U.S. Patent No. 9011770, entitled Device for Detecting Analytes in Fluid Samples. It protects a device for detecting the presence of an analyte in a liquid sample and having an opening for introducing the liquid sample into a first chamber for collecting the sample, a second chamber connected to the first chamber through a passageway and containing a test element, a third chamber connected to the second through a passageway and containing a plunger with a push bar that separates the third chamber into first and second zones, and then a lid for closing the opening and contacting the push bar to move the plunger, causing a volume of air to be drawn from the second chamber into the second zone of the third chamber, which itself causes a volume of the liquid sample to be drawn from a first chamber through the first passageway into the second chamber to contact the test element. The resulting invention provides a device through which the accurate testing of a subject can be easily performed by someone other than a trained healthcare professional.
Improvements to diagnostic testing devices which can identify diseases in a shorter amount of time than conventional testing devices are discussed within U.S. Patent No. 9134303, entitled ICT Immunoassay for Legionella pneumophila Serogroup 1 Antigen Employing Affinity Purified Antibodies Thereto. This protects a device for determining the presence of Legionella pneumophila serogroup 1 in a liquid sample, the device having a porous test strip with a sample receiving zone, multiple binding agents each having antigen specific affinity-purified polyclonal antibodies conjugated to a detectable particle and specifically binding an L. pneumophila antigen, multiple capture agents and a capture zone; the binding agents are disposed in a dried state along the flow path of the test strip from a receiving zone to a capture zone, but they are mobilizable by liquid flowing down the strip’s flow path. This invention protects an immunochromatic test (ICT) device which can test for the presence or absence of the bacteria causing Legionnaire’s disease within 15 minutes; similar tests require a few hours to return results properly. Alere has sought to widen the application of nucleic acid-based diagnostics in point-of-care and consumer testing with technologies like the one described in U.S. Patent No. 9157127, which is titled Monitoring Recombinase Polymerase Amplification Mixtures. It discloses a composition comprised of a mixture having a first population of particles including a recombinase, a single-strand DNA binding protein (SSB) and a first oligonucleotide having a first detectable label, and then a second population of particles including a recombinase, an SSB and a second oligonucleotide with a detectable label which is different from the first nucleotide. This diagnostic composition employs an isothermal method known as recombinase polymerase amplification (RPA) which can detect nucleic acids without the use of power-demanding instrumentation.
 Alere subsidiary eScreen has been focused on developments in managing patient assay results, as is evidenced by the issue of U.S. Patent No. 9189602, titled Electronic Custody and Control System for Human Assay Test Samples. This patent protects a non-transitory computer-readable storage medium with an executable program for assisting an entity in tracking the custody and control of a human analyte specimen provided by a donor by performing the steps of receiving donor information from the donor’s employer, storing the information identifying the donor in a donor database, receiving employer information and at least one test required by the employer to be performed on a specimen, storing employer information in an employer database, enabling a collector associated with a collection agency to access information on the donor, employer and test to be requested, receiving information identifying the unique identifier assigned to a specimen container, associating a computer-generated, forensically-defensible custody and control form (CCF) identifying the donor with the specimen container’s unique identifier, populating the CCF with at least some of the donor information, receiving an electronic signature from an electronic signature reader signed by the donor to verify accurate information on the CCF, storing that signature as part of the CCF, obtaining at least one test result for a test requested by an employer on a specimen, populating the CCF with the results and enabling the employer to access the CCF via an application. This innovation serves as an electronic storage system for CCFs, reducing the time-consuming nature of mailing paper CCFs as well as the expense of printing CCF documents.
Alere subsidiary eScreen has been focused on developments in managing patient assay results, as is evidenced by the issue of U.S. Patent No. 9189602, titled Electronic Custody and Control System for Human Assay Test Samples. This patent protects a non-transitory computer-readable storage medium with an executable program for assisting an entity in tracking the custody and control of a human analyte specimen provided by a donor by performing the steps of receiving donor information from the donor’s employer, storing the information identifying the donor in a donor database, receiving employer information and at least one test required by the employer to be performed on a specimen, storing employer information in an employer database, enabling a collector associated with a collection agency to access information on the donor, employer and test to be requested, receiving information identifying the unique identifier assigned to a specimen container, associating a computer-generated, forensically-defensible custody and control form (CCF) identifying the donor with the specimen container’s unique identifier, populating the CCF with at least some of the donor information, receiving an electronic signature from an electronic signature reader signed by the donor to verify accurate information on the CCF, storing that signature as part of the CCF, obtaining at least one test result for a test requested by an employer on a specimen, populating the CCF with the results and enabling the employer to access the CCF via an application. This innovation serves as an electronic storage system for CCFs, reducing the time-consuming nature of mailing paper CCFs as well as the expense of printing CCF documents.
We’ll wrap up our look at Alere’s patent portfolio with an exploration of a system for monitoring heart and kidney health and outlined within U.S. Patent No. 8969018, titled Methods and Compositions for Monitoring and Risk Prediction in Cardiorenal Syndrome. It claims a method of monitoring cardiorenal syndrome in a patient by performing an assay method that detects neutrophil gelatinase-associated lipocalin (NGAL) on a body fluid sample obtained from the patient, performing an assay method detecting B-type natriuretic peptide (BNP) on the same or different body fluid sample from the patient and relating the NGAL assay result and the BNP assay result to the patient’s cardiorenal syndrome status to assign an improving or worsening cardiorenal syndrome status to the patient. This innovation seeks to leverage the recent discovery that NGAL can be monitored as a new early marker for acute renal injury within two hours of an event, which could be useful in monitoring heart failure or renal dysfunction.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)


![[[Advertisement]]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-banner-early-bird-ends-Apr-21-last-chance-938x313-1.jpeg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
One comment so far.
Night Writer
February 17, 2016 09:00 amOT, but Tania Simoncelli has a TED talk (Jan 2016) about the ACLU bringing the Myriad case. Lots of things in it to object to, but well worth watching.