 Immunotherapy has emerged as one of the most promising mechanisms to combat diseases like cancer and microbial infections. Since 2000, multiple antibody drugs have reached blockbuster status, including the anti-TNF antibodies adalimumab and infliximab, the anti-CD20 antibody rituximab, the anti-VEGF-A antibody bevacizumab, and the anti-HER-2 antibody Trastuzumab. Ulrich Storz, Intellectual property issues of immune checkpoint inhibitors, mAbs, 8:1, 10-26, DOI: 10.1080/19420862.2015.1107688 (2016). In 2016, five of the top 10 pharmaceuticals were antibody drugs, with combined sales exceeding $45.8 billion. The cancer immunotherapy market is expected to reach nearly $120 billion.
Immunotherapy has emerged as one of the most promising mechanisms to combat diseases like cancer and microbial infections. Since 2000, multiple antibody drugs have reached blockbuster status, including the anti-TNF antibodies adalimumab and infliximab, the anti-CD20 antibody rituximab, the anti-VEGF-A antibody bevacizumab, and the anti-HER-2 antibody Trastuzumab. Ulrich Storz, Intellectual property issues of immune checkpoint inhibitors, mAbs, 8:1, 10-26, DOI: 10.1080/19420862.2015.1107688 (2016). In 2016, five of the top 10 pharmaceuticals were antibody drugs, with combined sales exceeding $45.8 billion. The cancer immunotherapy market is expected to reach nearly $120 billion.
Pharmaceutical research and development is a lengthy and expensive process. It is even more costly to research, develop, and manufacture large molecule pharmaceuticals than small molecule ones. Thus, it is essential to build up strong patent portfolios to protect innovative biotherapies. With billions of dollars in annual sales, the stakes for immunotherapy patent disputes are extremely high. See, e.g., Centocor Ortho Biotech, Inc. v. Abbott Labs., 636 F.3d 1341 (Fed. Cir. 2011) (invalidating Centocor’s asserted claims covering Abbott’s Humira® and reversing a jury award of $1.67 billion). This article is the opening of a series of articles, aimed at exploring the various aspects of patenting immunotherapies with a focus on antibody-based drugs. While this article provides an overview of immunotherapy and the general types of patent claims sought to protect immunotherapeutic inventions, follow-up articles will explore patent validity issues, such as written description and enablement, novelty and inventiveness, and patent eligibility, related to immunotherapy.
Overview of Immunotherapy
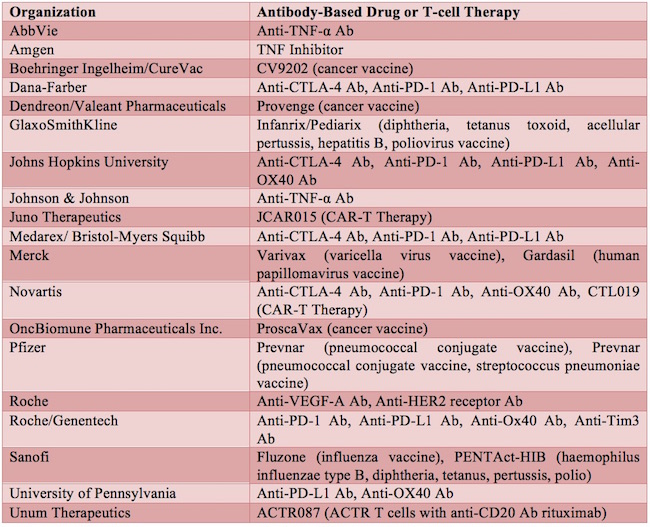
The commercial development of therapeutic monoclonal antibodies first began in the early 1980s. Dawn M Ecker et al., The therapeutic monoclonal antibody market, mAbs, 7:1, 9-14, DOI: 10.4161/19420862.2015.989042 (2015). Orthoclone OKT3 was the first therapeutic monoclonal antibody approved, in 1986, for the prevention of kidney transplant rejection. Id. Since then, therapeutic monoclonal antibodies, along with other antibody-related products, such as Fc-fusion proteins, antibody fragments, and antibody-drug conjugates, have emerged as one of the major classes of drugs in the biopharmaceutical field. Id. See Table 1 for a list of exemplary antibody drugs approved or in development.
Antibodies function by a number of mechanisms, including neutralization, blocking receptor-ligand interactions, antibody-dependent cell-mediated cytotoxicity, or complement-dependent cytotoxicity. The subsections below highlight four active areas currently in the immunotherapy field, namely, Immune Checkpoint Inhibitors (ICIs), Antibody-drug conjugates (ADCs), T-cell therapy, and bispecific antibodies (bsAbs).
A. Immune Checkpoint Inhibitors (ICIs)
The cancer immunotherapy market is expected to be led by ICIs, demonstrating the importance of this therapeutic approach. Tumor cells attempt to exploit this checkpoint system by regulating the immune response in order to enhance their potential for escape and survival. Storz. ICIs can interrupt this tumor suppression strategy by reactivating the immune system and, hence, reestablishing its capacity to combat tumors. Id. Two of the most common ICI targets for drug development have been the PD-1/PD-L1-PD-L2 and CTLA4/CD80-CD86 receptor-ligand signaling pathways. Both pathways result in inhibition of T-cell activation, proliferation, and survival. Whereas the CTLA-4 receptor is only expressed in T cells, PD-1 is expressed in activated T cells, B cells, and myeloid cells. Moreover, whereas CTLA-4 works in the priming phase of T-cell activation, PD-1 works later, during the effector phase, mostly in peripheral tissues. Consequently, combined inhibition of both of these pathways could produce synergistic effects that have been explored to treat cancer.
Today, more than six drugs utilize these two checkpoints. Some of the anti-CTLA-4 and anti-PD-1 antibodies have been approved for the treatment of unresectable or metastatic melanoma. Several candidates have been or are in Phase III clinical trials for head and neck, urothelial, lung, kidney, and prostate cancer (e.g., anti-CTLA-4 antibodies Ipilimumab and Tremelimumab), as well as gastric cancer, lung cancer, head and neck cancer, and urothelial and kidney cancer (e.g., anti-PD-1 antibodies Nivolumab and Pembrolizumab; anti-PD-L1 antibodies Avelumab, Durvalumab, and Atezolizumab). In addition, at least one study has investigated the biological effect of combined CTLA-4 and PD-1 blockade. Lydia Dyck et al., Immune checkpoints and their inhibition in cancer and infectious diseases, Eur. J. Immunol., 47:765-779, DOI: 10.1002/eji.201646875 (2017).
B. Antibody-Drug Conjugates (ADCs)
ADCs also emerged quite successfully in the antibody field. They consist of monoclonal antibodies linked to biologically active drugs, thus, in essence, combining the targeting ability of antibodies with the cytotoxic ability of other drugs. The development of companion diagnostics has greatly benefited immunotherapy and increased the success rate of several antibody-based therapies. This combinatory treatment has proven very effective for diseases, such as cancers. Some currently approved antibody-drug conjugates include inotuzumab and trastuzumab emtansine (T-DM1), as shown in Table 1. T-DM1 is also referred to as Kadcyla and consists of the monoclonal antibody trastuzumab linked to the cytotoxic agent emtansine. Kadcyla functions by targeting the HER2/neu receptor and cellular tubulin and has proven effective for treatment of a number of cancers, including breast cancer.
C. T-Cell Therapy
T-cell therapy has also proven effective and successful in the biopharmaceutical field. This type of therapy involves reprogramming a patient’s own immune T cells to attack tumors. One type of well-known T-cell therapy comprises adoptive transfer of chimeric antigen receptor (CAR) T-cells. The benefit of CAR T-cells is evident in that such cells can be engineered to target essentially any tumor-associated antigen. One example of a CAR T-cell therapy in development is JCAR015 from Juno Therapeutics. This therapy specifically targets CD19 and is directed for treatment of a variety of leukemia.
D. Bispecific Antibodies (bsAbs)
Bispecific antibodies are recombinant proteins that can bind to two different types of antigen at the same time. For example, a bsAb can be engineered to bind a cytotoxic cell and a tumor cell to be killed. That way, the bsAb brings the cytotoxic cell and the target tumor cell into close proximity and facilitates tumor treatment. Amgen’s blinatumomab (targeting CD19 and CD3) and Fresenius Biotech GmbH/TRiOn Pharma GmbH’s catumaxomab (targeting EpCAM and CD3) are two examples of approved bispecific antibodies. Yang et al., Bispecific Antibodies as a Development Platform for New Concepts and Treatment Strategies, Int. J. Mol. Sci., 18(48), DOI: 10.3390/ijms18010048 (2017). Several others are currently in clinical trials. Id.
Table 1 Exemplary Antibody-Based Drugs and T-cell Therapy Approved or in Development
Patent Claims Covering Immunotherapeutics
The promise of immunotherapy as a treatment option has opened up the intellectual property landscape of the field. Many companies and institutions have filed patent applications related to the various drugs and targets, such as those listed in Table 1, seeking to protect their immunotherapeutic inventions. Storz; Matthieu Collin, Immune checkpoint inhibitors: a patent review (2010-2015), Expert Opin. Ther. Pat., 26(5):555-564, DOI: 10.1080/13543776.1176150 (2016); http://www.biospace.com/News/top-10-best-selling-biotech-drugs/393360; http://blog.proclinical.com/top-selling-vaccines-in-history. For example, as of May 2017, at least 1377 patent families exist that recite both antibody and CTLA-4 in their claims. Storz at 12. Along those lines, about 500 patent families are directed to antibodies against the PD-1/PD-L1 interaction, about 350 patent families are directed to antibodies and OX40, and about 115 patent families are directed to antibodies and TIM3 or LAG 3. Collin; Storz. The United States Patent and Trademark Office (USPTO) cites 149 patents that claim some aspects of CTLA-4, 129 patents that claim some aspects of PD-1, 82 patents that claim some aspects of OX40, nine patents that claim some aspects of TIM3, and eight patents that claim some aspects of LAG 3.
The claim language in a patent is crucial to the effective protection of the invention, and the balance between breadth of the claims and validity is a difficult one to achieve. Nowhere has this balance come into play more frequently than in the cases of patenting antibody-related inventions. While the authors will explore validity or patentability issues in the follow-up articles, the section below provides an overview of the types of claims that one can use as a guide to build up a patent portfolio. For example, antibody-related inventions can be protected through various claim types such as (1) antibody per se, (2) combinations, (3) first medical use, (4) second medical use, (5) formulations, (6) administration, (7) dosage forms, (8) dosage regimens, (9) purification methods, (10) generation methods, and (10) patient selection/companion diagnostics. The following illustrates some common claim types to cover antibody-based inventions.
1. Claims to the Antibody Itself
a. An antibody that binds to protein X.
b. An antibody that binds to protein X, wherein the antibody has a dissociation constant (Kd) of 10-9 (or any other functional property such as neutralizing activity, agonistic/antagonistic activity, or immune action/antibody-dependent cell-mediated cytotoxicity (ADCC)/complement-dependent cytotoxicity (CDC).
c. An antibody that binds to an epitope comprising the sequence of SEQ ID NO: X.
d. An antibody against protein X, or a protein-X-binding fragment thereof, wherein the antibody comprises complementary determining region (CRD)-H1 corresponding to residues x1-y1 of SEQ ID NO: X, CDR-H2 corresponding to residues x2-y2 of SEQ ID NO: X, and CDR-H3 corresponding to residues x3-y3 of SEQ ID NO: X for the heavy chain CDRs, and CDR-L1 corresponding to residues x4-y4 of SEQ ID NO: X, CDR-L2 corresponding to residues x5-y5 of SEQ ID NO: X, and CDR-L3 corresponding to residues x6-y6 of SEQ ID NO: X for the light chain CDRs.
e. An antibody-drug conjugate having the formula Ab-X, wherein the antibody is the antibody of any one of claims 1a.-1e. and X comprises Y.
2. Claims to Compositions
a. A pharmaceutical composition comprising the antibody according to any one of claims 1a.-1e. and a pharmaceutically acceptable carrier or diluent.
b. A pharmaceutical composition comprising the antibody according to any one of claims 1a.-1e. and a pharmaceutically acceptable carrier or diluent, wherein the antibody is glycosylated in one or both chains.
c. A pharmaceutical composition comprising the antibody according to any one of claims 1a.-1e. and a pharmaceutically acceptable carrier or diluent, wherein the composition has a certain physical/chemical property (e.g., shelf life, purity, and percent aggregation).
d. A pharmaceutical composition comprising the antibody according to any one of claims 1a.-1e. in combination with drug X.
e. A pharmaceutical composition comprising the antibody according to any one of claims 1a.-1e. and a given sugar, buffer, salt, stabilizer, surfactant, adjuvant, etc.
3. Claims to a Nucleic Acid Encoding the Antibody
a. An isolated nucleic acid encoding the antibody produced by hybridoma X.
b. An isolated nucleic acid encoding the heavy chain and/or light chain variable region of antibody X, wherein the heavy chain/light variable region comprises SEQ ID NO: X.
4. Claims to Methods of Using the Antibody
a. A method of treating disease X in a subject in need thereof, wherein the method comprises administering to the subject a therapeutically effective amount of the antibody of any one of claims 1a.-1e.
b. A method of treating disease X in a subject in need thereof, wherein the method comprises administering to the subject a therapeutically effective amount of a pharmaceutical composition of any one of claims 2a.-2e.
c. A method of treating disease X in a subject in need thereof, wherein the method comprises administering to the subject the antibody-drug conjugate of claims 1e.
d. A method of treating disease X in a subject in need thereof, wherein the method comprises administering to the subject the antibody at a certain dosage regimen.
e. A method of treating disease X in a subject in need thereof, wherein the method comprises administering to the subject the antibody via a certain route of administration (IV, IM, SC, or others).
f. A method of treating disease X in a subject in need thereof comprising administering drug Y to the subject, wherein the subject has been diagnosed with disease X using the antibody of any one of claims 1a.-1e. prior to treatment.
g. A method of diagnosing disease X in a subject, comprising reacting a sample from the subject with an antibody Y against antigen W and detecting the binding of antibody Y to antigen W, wherein the presence of binding indicates that the subject suffers from disease X.
The above is not an exhaustive list of claims related to immunotherapy. To build up a strong patent portfolio covering immunotherapeutic inventions, as with other types of inventions, a key is to craft a variety of claims with variations in claim types and scopes. In the biopharmaceutical field, the patent case law has not been developed as much as small molecule-based therapies. As case law is more unpredictable in the biopharmaceutical area, it is even more important to have a variety of patent claims to cover immunotherapeutic inventions from various aspects.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)



![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
No comments yet.