 On Tuesday, November 20th, the Court of Appeals for the Federal Circuit issued a nonprecedential decision in Indivior Inc. v. Dr. Reddy’s Laboratories, S.A., which vacated a preliminary injunction handed out by the District of New Jersey in a Hatch-Waxman patent infringement case brought by British pharmaceutical firm Indivior. The majority panel of Circuit Judges Alan Lourie and Kara Stoll found that the district court erred in the interpretation of the scope of patent claims asserted by Indivior. Circuit Judge Pauline Newman authored a dissenting opinion in which she explained she would have found the district court’s preliminary injunction grant sustained on appeal.
On Tuesday, November 20th, the Court of Appeals for the Federal Circuit issued a nonprecedential decision in Indivior Inc. v. Dr. Reddy’s Laboratories, S.A., which vacated a preliminary injunction handed out by the District of New Jersey in a Hatch-Waxman patent infringement case brought by British pharmaceutical firm Indivior. The majority panel of Circuit Judges Alan Lourie and Kara Stoll found that the district court erred in the interpretation of the scope of patent claims asserted by Indivior. Circuit Judge Pauline Newman authored a dissenting opinion in which she explained she would have found the district court’s preliminary injunction grant sustained on appeal.
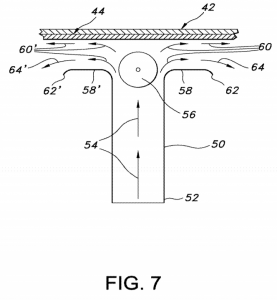
The patent-at-issue in this case is U.S. Patent No. 9931305, titled Uniform Films for Rapid Dissolve Dosage Form Incorporating Taste-Making Compositions. Issued this April, it covers a drug delivery composition for making individual unit doses in self-supporting film-dosage form, the individual doses being cut from a self-supporting continuously cast film containing a desired amount of an active drug composition uniformly distributed throughout the film. The ‘305 patent shares a specification with Invidior’s U.S. Patent No. 8603514, same title as the ‘305 patent.
The ‘514 patent was asserted by Invidior in a previous Hatch-Waxman suit against Dr. Reddy’s in the District of Delaware after Dr. Reddy’s filed an abbreviated new drug application (ANDA) with the U.S. Food and Drug Administration (FDA) for approval of a generic version of Indivior’s Suboxone Film, a treatment for opioid dependency. In that case, Indivior’s patent infringement claims failed after the district court determined that the ‘514 patent disavowed the sole use of conventional air drying across the top of the film, finding that the specification disclaimed and disparaged this method, and determined that Dr. Reddy’s generic film product was dried through conventional means and thus didn’t infringe the ‘514 patent.
After the Delaware court found non-infringement of the ‘514 patent, Indivior amended claims of a pending patent application that ultimately issued as the ‘305 patent to replace the terms “dried” and “drying” with “continuously” and “continuously cast.” Indivior also filed a terminal disclaimer on the ‘305 patent to overcome obviousness-type double patenting rejections over the ‘514 patent. The same day the ‘305 patent issued, Indivior filed another Hatch-Waxman suit against Dr. Reddy’s in the District of New Jersey. Dr. Reddy’s launched its generic film product the same day that the FDA approved its ANDA and Indivior quickly moved for a temporary restraining order and a preliminary injunction, both of which were granted by the district court.
On appeal, the Federal Circuit majority concluded that the district court had abused its discretion in granting the injunction. The panel majority found that the ‘305 patent’s specification disparaged, and therefore disclaimed, the method of drying the films with the use of conventional methods which only dry the top of the film. Indivior argued that the amended claims in the ‘305 patent overcome specification disclaimer because “dried” or “drying” had no textual basis in the claims. The Federal Circuit majority, however, found both that the claims did have a textual basis and, even if they didn’t, case law precedent didn’t require such a basis when the specification made such a disclaimer clear.
Further, the Federal Circuit majority found that claim preclusion likely barred Indivior’s suit as the claims of the ‘305 patent were patentably indistinct from the claims of the ‘514 patent. The appellate court found that the parties and accused products in this case were the same as the Delaware case, which reached a final judgment on the merits. The terminal disclaimer filed by Indivior on the ‘305 patent provided the Federal Circuit with “a strong clue” that the claims of either patent were patentably indistinct.
In her dissent, Judge Newman determined that the district court’s grant of a preliminary injunction was imposed “on full and careful analysis of law and equity.” A grant of preliminary injunction is meant to preserve the relative position of parties in opposition until a trial can be held and Dr. Reddy’s decision to enter the market while the Hatch-Waxman suit was ongoing was made with the knowledge that an injunction might be entered against it. Judge Newman argued that her colleagues weren’t properly considering the district court’s equitable discretion and rather made findings on the merits of infringement before a trial on infringement was conducted. Further, Judge Newman found that the majority improperly read the drying limitation into the claims of the ‘305 patent which covered not a drying method but a film for transmucosal administration of an active ingredient. The majority’s decision imported the drying limitation from the ‘514 patent claims into the ‘305 patent claims despite the fact that the ‘305 patent was amended specifically to remove this limitation.
Judge Newman also determined that the majority’s finding on claim preclusion erroneously treated the Delaware case on the ‘514 patent as barring a suit brought on the ‘305 patent, which has different claims. Multiple Federal Circuit decisions had previously found that a terminal disclaimer doesn’t cause all claims of a first patent to be patentably indistinct with the claims of the second patent. “Imposing irreparable harm on Indivior looms over the panel majority’s vacatur of the preliminary injunction based in part on a judgment currently pending appeal,” Judge Newman wrote.
Finally, Judge Newman took issue with the panel majority’s decision to decline review of the equitable factors which the district court took into account when granting the preliminary injunction. Where the majority found that it didn’t need to reach the aspects of the district court’s discretionary ruling because of its determination on the merits of the infringement claim, Judge Newman found that “the balancing of all factors is the foundation of a discretionary ruling” such as the one that had been appealed in this case. These factors include the potential that Indivior would lose market share and that the generic film would impair Indivior’s research and development, both of which would irreparably harm Indivior while the harm to Dr. Reddy’s could be monetized in the event that Indivior’s infringement suit was unsuccessful. The district court also found that the public interest would be served by the injunction because it wouldn’t deny access to the active ingredient, which can be administered by other means readily available on the market.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[[Advertisement]]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-2024-banner-938x313-1.jpeg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
One comment so far.
EG
December 7, 2018 07:33 amHey Steve,
As usual, Newman is right, as she often is in dissent. Obtaining a permanent injunction, much less a preliminary injunction after eBay is about as rare as finding a unicorn.