 The Federal Circuit recently reversed a decision by the United States District Court for the District of Delaware holding that a patent filed after the Uruguay Round Agreement Act (“URAA”) is a proper obviousness-type double patenting reference against an earlier-filed, yet later-expiring, pre-URAA patent. Applying the Federal Circuit case Gilead Sciences, Inc. v. Natco Pharma Ltd., the district court invalidated the earlier-filed compound patent by asserting the later-filed method of treatment patent as a double patenting reference. The Federal Circuit reversed the decision by holding the analysis in Gilead “was limited to the context of when both patents in question are post-URAA patents.” While the Court limited the present opinion to the specific facts of this case, the Court applied pre-URAA double-patenting practices to the pre-URAA patent and reasoned that the invalidating reference “did not exist as a double patenting reference” when the pre-URAA patent issued. Novartis Pharms. Corp. v. Breckenridge Pharm. Inc., Nos. 2017-2173, 2017-2175, 2017-2176, 2017-2178, 2017-2179, 2017-2180, 2017-2182, 2017-2183, 2017-2184, 2018 (Fed. Cir. Dec. 7, 2018) (Before Prost, Chief Judge, Wallach, and Chen, Circuit Judges) (Opinion for the Court by Chen, Circuit Judge).
The Federal Circuit recently reversed a decision by the United States District Court for the District of Delaware holding that a patent filed after the Uruguay Round Agreement Act (“URAA”) is a proper obviousness-type double patenting reference against an earlier-filed, yet later-expiring, pre-URAA patent. Applying the Federal Circuit case Gilead Sciences, Inc. v. Natco Pharma Ltd., the district court invalidated the earlier-filed compound patent by asserting the later-filed method of treatment patent as a double patenting reference. The Federal Circuit reversed the decision by holding the analysis in Gilead “was limited to the context of when both patents in question are post-URAA patents.” While the Court limited the present opinion to the specific facts of this case, the Court applied pre-URAA double-patenting practices to the pre-URAA patent and reasoned that the invalidating reference “did not exist as a double patenting reference” when the pre-URAA patent issued. Novartis Pharms. Corp. v. Breckenridge Pharm. Inc., Nos. 2017-2173, 2017-2175, 2017-2176, 2017-2178, 2017-2179, 2017-2180, 2017-2182, 2017-2183, 2017-2184, 2018 (Fed. Cir. Dec. 7, 2018) (Before Prost, Chief Judge, Wallach, and Chen, Circuit Judges) (Opinion for the Court by Chen, Circuit Judge).
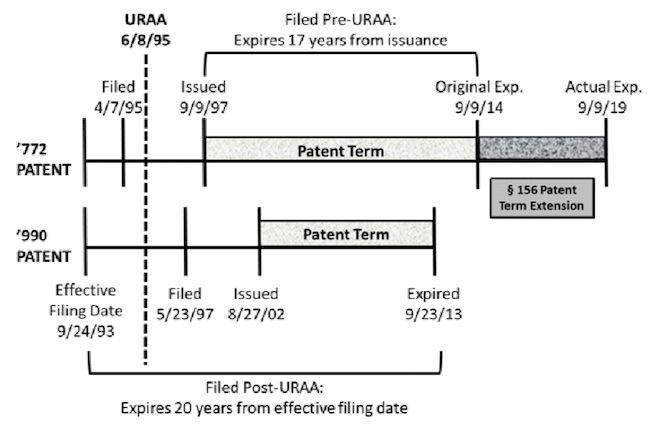
Novartis owns U.S. Patent No. 5,665,772 (the ’772 patent) for the compound everolimus—a cancer-treatment drug—and U.S. Patent No. 6,440,990 (the ’990 patent) directed to methods of treatment using everolimus. Novartis sued the Defendant drug manufacturers in the case when the Defendants sought FDA approval of generic versions of everolimus-based drugs. In the district court, the Defendants conceded that the proposed generic drugs infringed the claims of the ’772 patent. The parties also stipulated that if the ’990 patent was found to be a proper double patenting reference, the ’772 would be invalid for obviousness-type double patenting.
The timeline of the two patents is of particular importance to the case. Novartis filed the ’772 patent application on April 7, 1995. Two months later, on June 8, 1995, the URAA became effective. The URAA transition statute provided that applications filed before June 8, 1995, shall have a term of the greater of (1) 20 years from the effective filing date and (2) 17 years from issuance. As a result, the ’772 patent enjoyed a patent term of 17 years from its date of issuance, expiring on September 9, 2014. Although the ’772 patent also received a five-year term extension, the term extension did not affect the holding of the case.
The ’990 patent was filed on May 23, 1997, well after the effective date of the URAA. The term of the ’990 patent extended 20 years from the effective filing date of September 24, 1993, or until September 24, 2013. Accordingly, the ’990 patent, or the method of treatment for the ’772 patent, expired a full year before the ’772 patent, excluding the ’772 patent’s term extension. The Federal Circuit’s decision provided the following chart illustrating the important dates of the two patents:
Relying on Gilead, the district court found the post-URAA, later-filed ’990 patent to be a proper double patenting reference against the pre-URAA ’772 patent, thereby invalidating the asserted claims of ’772 patent. Novartis appealed the decision.
The narrow question presented to the Federal Circuit was whether “a post-URAA patent that issues after and expires before a pre-URAA patent [can] qualify as a double patenting reference against the pre-URAA patent.” The Federal Circuit first discussed the history of the judicially-created doctrine of obviousness-type double patenting. In particular, the Court discussed how pre-URAA law looked at the issuance dates of the patents to determine if a patentee was attempting to prolong the 17-year exclusivity of a patent. The Court discussed how Gilead recognized the change in patent law away from focusing on issuance dates, as issuance dates no longer “serve as reliable stand-ins for the expiration date of the patent.” The Court disagreed with the Defendant’s/Appellee’s position, however, that Gilead controlled in the present case.
The Court distinguished Gilead by clarifying that the case involved two post-URAA patents, or rather, both the invalidating patent and the invalid patent were filed after June 8, 1995. The Gilead analysis “was rooted in the consequences that flow from the implementation of the URAA’s new patent term rule.” In particular, patent law post-URAA allowed a certain degree of gamesmanship during prosecution, where a patentee could create a separate “chain” of applications having a longer term by claiming priority back to a later-filed application within a family.
The Court acknowledged that no such gamesmanship was present here because the ’772 patent expired “after the ’990 patent only due to happenstance of an intervening change in patent term law.” The ’990 patent had not issued when the ’772 patent issued, and the ’772 patent “was confined to a 17-year patent term.”
The Court also distinguished the case AbbVie, Inc. v. Mathilda & Terence Kennedy Institute of Rheumatology Trust, 764 F.3d 1366 (Fed. Cir. 2014), on similar grounds, recognizing that the case also involved two post-URAA patents.
Finally, the Court held that “where we have an earlier-filed, earlier-issued, pre-URAA patent that expires after the later-filed, later-issued, post-URAA patent,” the Court will “apply our traditional, pre-URAA obviousness-type double patenting practice.” It should be noted, however, that the Court highlighted in a footnote that other hybrid situations may exist, and the current opinion is limited to the specific facts of the case. The Court then looked to the ’772 patent’s issuance date as the reference for an obviousness-type double patenting analysis. Under the analysis, the ’990 patent is not a proper obviousness-type double patenting reference for the ’772 patent because, “[w]hen the ’772 patent issued, the ’990 patent had not yet issued and thus did not exist as a double patenting reference against the ’772 patent.”
The Court held this decision was in the spirit of the URAA transition statute, providing a patentee with the greater of 17 years from issuance or 20 years from the effective filing date, and the holding is consistent with preventing gamesmanship because the ’772 patent would have the same term regardless of whether Novartis obtained the ’990 patent.
Accordingly, by holding the ’990 patent was not a proper obviousness-type double patenting reference against the ’772 patent, the Federal Circuit reversed the district court’s decision invaliding the asserted claims of the ’772 patent.
Take Away
The obviousness-type double patenting analysis of Gilead Sciences, Inc. v. Natco Pharma Ltd is limited to multiple patents filed after the effective date of the Uruguay Round Agreement Act (URAA). When one patent is a pre-URAA patent, and another patent is a post-URAA patent, the analysis is different and may involve looking to the pre-URAA patent’s issuance date, depending on the facts of the case.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)



![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
No comments yet.