
“Boston Scientific Corporation sign” by D Coetzee is licensed under CC BY 2.0.
The Boston Scientific Corporation (NYSE: BSX), officially headquartered in Marlborough, MA, is a developer and manufacturer of medical device products. Its devices are used in a diverse set of medical specialities including neuromodulation, vascular surgery, oncology, radiology and interventional cardiology.
Boston Scientific’s research and development goals are aided by a network of three innovation centers for developing products as well as six global locations of the Boston Scientific Institute for Advancing Science, which offer product and procedure training programs. Last May, the company announced that it was joining the Optum Labs, a healthcare product and medical data analysis innovation initiative co-founded by the Mayo Clinic and UnitedHealth Group. In 2014, total R&D expenditures for Boston Scientific came to $816 million, or 11.4 percent of its revenues that year.
The most recent quarterly earnings report for Boston Scientific indicated that corporate revenues grew by 2.7 percent year-over-year to $1.887 billion in the latest quarter. Much of this increase was the result of 10 percent year-over-year growth in the company’s interventional cardiology division, the corporation’s largest business division. The company is still facing a great challenge, however, in the $7.2 billion lawsuit filed against Boston Scientific by Johnson & Johnson currently being tried in U.S. district court.
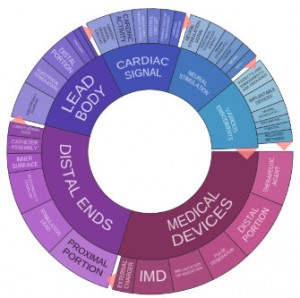 Boston Scientific did not make the IFI Claims list for the top 50 companies receiving patent grants in 2014 from the U.S. Patent and Trademark Office. In 2013, however, the company was 40th overall with 904 patent grants from the PTO, just ahead of medical device competitor Medtronic that year. Our research through Innography patent analytic tools shows us that the company did increase its U.S. patent grant earnings in 2014 to 943 U.S. patents, which would actually place it 37th among the IFI Claims list linked above; most of these are assigned to either Boston Scientific Scimed of Maple Grove, MN, or Boston Scientific Neuromodulation Corporation of Valencia, CA. Beyond medical devices, according to Innography data (see text cluster image right) we’re seeing innovations related to cardiac signals and neural stimulation reflected in the company’s recent patents.
Boston Scientific did not make the IFI Claims list for the top 50 companies receiving patent grants in 2014 from the U.S. Patent and Trademark Office. In 2013, however, the company was 40th overall with 904 patent grants from the PTO, just ahead of medical device competitor Medtronic that year. Our research through Innography patent analytic tools shows us that the company did increase its U.S. patent grant earnings in 2014 to 943 U.S. patents, which would actually place it 37th among the IFI Claims list linked above; most of these are assigned to either Boston Scientific Scimed of Maple Grove, MN, or Boston Scientific Neuromodulation Corporation of Valencia, CA. Beyond medical devices, according to Innography data (see text cluster image right) we’re seeing innovations related to cardiac signals and neural stimulation reflected in the company’s recent patents.
[Bio-Pharma]
Boston Scientific’s Issued Patents: Implantable Devices for Treating Disorders from Depression to Urinary Incontinence
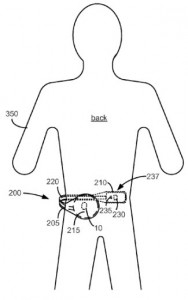 We were able to find some intriguing inventions related to implantable medical devices and systems, including the simpler system for providing power to device implants inside of a patient discussed by U.S. Patent No. 8886333, which is titled Self-Affixing External Charging System for an Implantable Medical Device. The charging system claimed here contains a flexible support coupled to a first module comprising a coil as well as a second module containing circuitry for activating the coil to produce a charging field. This improved means for attaching the external charger to a patient is capable of charging devices wherever they are implanted in a patient without becoming cumbersome while the patient is walking or otherwise moving.
We were able to find some intriguing inventions related to implantable medical devices and systems, including the simpler system for providing power to device implants inside of a patient discussed by U.S. Patent No. 8886333, which is titled Self-Affixing External Charging System for an Implantable Medical Device. The charging system claimed here contains a flexible support coupled to a first module comprising a coil as well as a second module containing circuitry for activating the coil to produce a charging field. This improved means for attaching the external charger to a patient is capable of charging devices wherever they are implanted in a patient without becoming cumbersome while the patient is walking or otherwise moving.
Implantable devices for treating neurological conditions are protected by a pair of patents we came across today. A treatment for depression that involves the application of electrical stimulation energy is the focus of U.S. Patent No. 8914120, issued under the title Method for Treating Depression by Indirectly Stimulating Raphe Nuclei. The method for treating a patient suffering from depression protected here involves applying electrical stimulation energy from at least one electrode implanted in an epidural space of a patient to nerve fibers leading to the patient’s hypothalamus. This treatment is intended to stimulate the generation and metabolism of serotonin released by the raphe nuclei located in a patient’s mid-brain. Improved systems for applying electrical energy to a patient 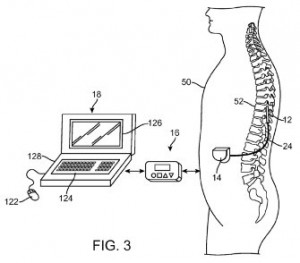 through an implanted device to aid in pain reduction therapies is described within U.S. Patent No. 8868193, entitled Neurostimulation System and Method for Providing Therapy to a Patient With Minimal Side Effects. This patent protects a method of providing therapy to a tissue region of a patient using multiple electrodes by conveying an electrical pulsed waveform from the first electrode along a sensory neural fiber and conveying electrical energy between the second electrode and a blocking site of the spinal cord. The technology is designed to reduce side effects, such as involuntary movements, in patients receiving electrical neurostimulation along the spinal cord to treat pain.
through an implanted device to aid in pain reduction therapies is described within U.S. Patent No. 8868193, entitled Neurostimulation System and Method for Providing Therapy to a Patient With Minimal Side Effects. This patent protects a method of providing therapy to a tissue region of a patient using multiple electrodes by conveying an electrical pulsed waveform from the first electrode along a sensory neural fiber and conveying electrical energy between the second electrode and a blocking site of the spinal cord. The technology is designed to reduce side effects, such as involuntary movements, in patients receiving electrical neurostimulation along the spinal cord to treat pain.
As we mentioned earlier, cardiovascular treatments and device tech has been a recent bright spot for Boston Scientific so we wanted to profile a novel treatment for atrial fibrillation featured within U.S. Patent No. 8951247, which is titled Methods and Apparatus for Forming Cardiac Lesions and Assessing Lesion Quality. This patent claims a method of treating a condition of the heart by introducing a catheter with a braided conductive member into the heart, forming a lesion in the heart with the conductive member, generating a pacing signal at a catheter electrode and detecting a received signal indicative of a lesion quality at a second catheter electrode. The invention is intended to improve the use of catheter ablation for treating atrial fibrillation by forming a lesion in the heart that alter’s the heart’s conductive properties.
We also took some time to explore a couple of patents related to the treatment of certain pelvic disorders, especially those experienced more commonly by women. U.S. Patent No. 8939886, entitled Systems, Methods and Devices Relating to a Removable Sleeve for an Implantable Sling, outlines the use of an implantable device to aid in the treatment of stress urinary incontinence in women. The sling assembly protected here includes an implantable sling sized and shaped to provide a urethral platform and a removable sleeve with multiple flapped portions which are configured to facilitate the removal of the removable sleeve from the implantable sling. The innovation allows for the delivery of a sling while c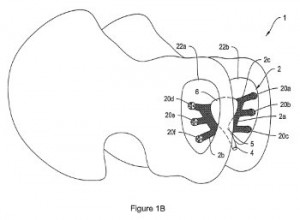 reating only one incision into a patient, reducing the patient trauma caused by other conventional methods of implanting a sling. Urinary incontinence and other pelvic area disorders are addressed by the invention protected by U.S. Patent No. 8944989, issued under the title Systems, Devices, and Methods for Treating Pelvic Floor Disorders. The patent claims a method of treating urinary incontinence in a patient by providing a mesh implant with anchors, securing the anchors to tissues within a patient and positioning the mesh material of the implant so that it is posterior to a patient’s bladderneck. This mesh implant can also be implanted through the use of a single incision, which again reduces patient trauma.
reating only one incision into a patient, reducing the patient trauma caused by other conventional methods of implanting a sling. Urinary incontinence and other pelvic area disorders are addressed by the invention protected by U.S. Patent No. 8944989, issued under the title Systems, Devices, and Methods for Treating Pelvic Floor Disorders. The patent claims a method of treating urinary incontinence in a patient by providing a mesh implant with anchors, securing the anchors to tissues within a patient and positioning the mesh material of the implant so that it is posterior to a patient’s bladderneck. This mesh implant can also be implanted through the use of a single incision, which again reduces patient trauma.
[Companies-1]
Patent Applications of Note: More Implantable Devices as well as Pulmonary Disease Treatments
The lungs are among the many areas of the human body targeted by Boston Scientific medical devices, especially for the treatment of chronic obstructive pulmonary disease (COPD). A minimally invasive procedure that encourages expansion of healthier lung portions 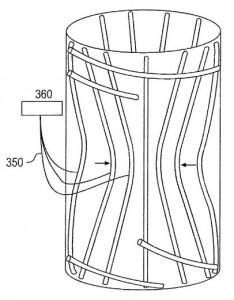 would be protected by U.S. Patent Application No. 20140324094, titled Devices for Obstructing Passage of Air or Other Contaminants Into a Portion of a Lung and Methods of Use. The patent application claims a device for selectively controlling air flow that includes an elongate member having a proximal end, a distal end and a lumen extending between the two and an inner member configured to adjust the diameter of a portion of the lumen. This treatment addresses the shortcomings of conventional techniques like lung volume reduction surgery or lung transplantation, complicated procedures which can create a great deal of patient discomfort. Other treatment methods for reducing the volume of a lung’s diseased region are at the center of U.S. Patent Application No. 20150025629, entitled Medical Device, System, and Method for Regulating Fluid Flow in Bronchial Passageways. The medical device claimed here includes an elongate tubular member with a plurality of channels extending between proximal and distal ends, multiple extensions from the device’s distal end which are configured to be placed in different lung passageways and a valve member configured to prevent fluid flow in various sets of channels. This invention results in a device that achieves better regulation of air flowing in and out of diseased lung portions.
would be protected by U.S. Patent Application No. 20140324094, titled Devices for Obstructing Passage of Air or Other Contaminants Into a Portion of a Lung and Methods of Use. The patent application claims a device for selectively controlling air flow that includes an elongate member having a proximal end, a distal end and a lumen extending between the two and an inner member configured to adjust the diameter of a portion of the lumen. This treatment addresses the shortcomings of conventional techniques like lung volume reduction surgery or lung transplantation, complicated procedures which can create a great deal of patient discomfort. Other treatment methods for reducing the volume of a lung’s diseased region are at the center of U.S. Patent Application No. 20150025629, entitled Medical Device, System, and Method for Regulating Fluid Flow in Bronchial Passageways. The medical device claimed here includes an elongate tubular member with a plurality of channels extending between proximal and distal ends, multiple extensions from the device’s distal end which are configured to be placed in different lung passageways and a valve member configured to prevent fluid flow in various sets of channels. This invention results in a device that achieves better regulation of air flowing in and out of diseased lung portions.
Implantable devices were also heavily reflected in the patent applications recently filed by Boston Scientific with the USPTO, according to what we saw in our latest survey. Tools for harnessing alternative forms of energy to charge an implanted device are explained by U.S. Patent Application No. 20140354211, entitled Solar-Powered External Charger and Solar-Powered External Charger Cradle for Medical Implantable Device Systems. By providing a means for generating device energy from solar power, this invention aims to address problems in charging a patient’s medical device while outdoors or during an electricity outage. The patent application would protect an external charger for providing power to an implantable medical device which includes a housing chamber containing a charging coil producing a magnetic field to provide power to a device, a battery for providing power to the charging coil, a charge 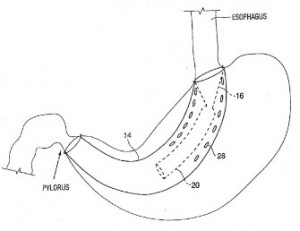 circuit that provides a charging current to the battery and a solar cell configured to produce a first voltage for the charge circuit. An implantable satiation device designed to encourage weight loss in patients is the focus of U.S. Patent Application No. 20140364792, filed under the title Gastro-Esophageal Implants. The implant for a patient that would be protected here includes a sleeve extending from an upstream to a downstream opening and configured to be secured with a gastro-esophageal junction region so that the upstream opening is aligned with the esophagus and an elongate tube which extends into the small intestine. This implantable device is intended to overcome the obstacles in traditional medical procedures for controlling obesity, such as complex gastric pouch procedures or implantation of gastric balloons, which can unintentionally migrate along the gastrointestinal tract. Implantable tools for providing better control of active bleeding in gastrointestinal systems is featured within
circuit that provides a charging current to the battery and a solar cell configured to produce a first voltage for the charge circuit. An implantable satiation device designed to encourage weight loss in patients is the focus of U.S. Patent Application No. 20140364792, filed under the title Gastro-Esophageal Implants. The implant for a patient that would be protected here includes a sleeve extending from an upstream to a downstream opening and configured to be secured with a gastro-esophageal junction region so that the upstream opening is aligned with the esophagus and an elongate tube which extends into the small intestine. This implantable device is intended to overcome the obstacles in traditional medical procedures for controlling obesity, such as complex gastric pouch procedures or implantation of gastric balloons, which can unintentionally migrate along the gastrointestinal tract. Implantable tools for providing better control of active bleeding in gastrointestinal systems is featured within 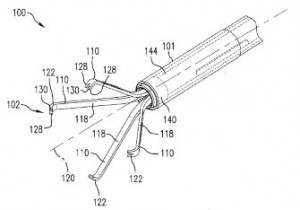 U.S. Patent Application No. 20150018848, which is titled Tissue Grasping and Wound Closing/Hemostasis/Clipping Device. The device for clipping tissue claimed here includes a clip with multiple arms and a slidable pusher member that actuates a tissue gripping configuration. This clip could be used to close openings in certain tissues or to compress lung tissues for treating COPD.
U.S. Patent Application No. 20150018848, which is titled Tissue Grasping and Wound Closing/Hemostasis/Clipping Device. The device for clipping tissue claimed here includes a clip with multiple arms and a slidable pusher member that actuates a tissue gripping configuration. This clip could be used to close openings in certain tissues or to compress lung tissues for treating COPD.
Ablation techniques for the kidney to treat certain patient conditions are explained by U.S. Patent Application No. 20140378967, which is titled Medical Devices for Renal Nerve Ablation Having Rotatable Shafts. This patent would protect a medical device for renal nerve ablation which includes a catheter shaft with a first lumen, an expandable member coupled to distal regions of an outer and inner shaft, which are relatable to each other, and one or more active electrodes on the expandable member’s exterior surface. This technology is intended to improve the ablation of renal nerves, which is a technique used to treat high blood pressure, while reducing the side effects created by conventional means of renal nerve ablation.
Finally today, we’ll sign off after a look at another cardiovascular therapy which makes use of catheters for the treatment of atrial fibrillation. U.S. Patent Application No. 20150018811, which is titled Regulating Pressure to Lower Temperature in a Cryotherapy Balloon Catheter, discusses methods of ablating heart and pulmonary vein tissues through the use of extreme cooling. The method of operating a cryotherapy balloon catheter claimed here involves introducing a cryotherapy balloon catheter to a treatment site within a patient’s body, regulating a flow of cryogenic fluid to and from the balloon through the use of an elongated member to create a first pressure and temperature within the balloon and then further regulating the cryogenic fluid to create a second pressure and temperature, both of which are less than the first pressure and temperature. This ablation technique is similar to the patented atrial fibrillation treatment discussed in the patent section above, both of which are designed to eliminate aberrant electrical signals causing atrial fibrillation.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
No comments yet.