 Although the Constitution provides inventors with certain “exclusive Right[s] to their … Discoveries,” there are limits to the scope of eligibility for such discoveries under the patent system. Subject matter eligibility is defined in 35 U.S.C. § 101 as “any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof.” Case law has established several exceptions to eligible subject matter, namely, laws of nature, natural phenomena, and abstract ideas. Although laws of nature, natural phenomena, and abstract ideas are not themselves patentable, their application or use has the potential to be patent eligible. Unfortunately for practitioners, these standards are vague, creating uncertainty that puts inventors, companies, and investors in a difficult situation – particularly in an industry like the biotechnology industry where research and development costs are high. Inventors, companies, and investors must determine whether to devote substantial funds and efforts into a discovery that may or may not qualify for patent protection. If the resulting invention is ultimately deemed to be a sufficient application of a law of nature, natural phenomenon, or abstract idea, the investment in research and development may be recovered during the period of exclusivity provided by the patent system. However, if the invention is deemed to be a mere law of nature, natural phenomenon, or abstract idea, competitors will be able to influence pricing decisions and control a market share, so as to inhibit the ability of an inventor, company, or investor to recoup its investment. Such uncertainty has the potential to slow progress in the industry.
Although the Constitution provides inventors with certain “exclusive Right[s] to their … Discoveries,” there are limits to the scope of eligibility for such discoveries under the patent system. Subject matter eligibility is defined in 35 U.S.C. § 101 as “any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof.” Case law has established several exceptions to eligible subject matter, namely, laws of nature, natural phenomena, and abstract ideas. Although laws of nature, natural phenomena, and abstract ideas are not themselves patentable, their application or use has the potential to be patent eligible. Unfortunately for practitioners, these standards are vague, creating uncertainty that puts inventors, companies, and investors in a difficult situation – particularly in an industry like the biotechnology industry where research and development costs are high. Inventors, companies, and investors must determine whether to devote substantial funds and efforts into a discovery that may or may not qualify for patent protection. If the resulting invention is ultimately deemed to be a sufficient application of a law of nature, natural phenomenon, or abstract idea, the investment in research and development may be recovered during the period of exclusivity provided by the patent system. However, if the invention is deemed to be a mere law of nature, natural phenomenon, or abstract idea, competitors will be able to influence pricing decisions and control a market share, so as to inhibit the ability of an inventor, company, or investor to recoup its investment. Such uncertainty has the potential to slow progress in the industry.
Within the last several years, the Supreme Court has weighed in multiple times as to where the line falls between a mere exception and a patentable application of a law of nature, natural phenomenon, or abstract idea. These decisions invalidated at least some contested claims as directed to an exception and lacking sufficient other features to qualify as subject matter eligible for patentability.
Specifically:
- In March 2012, the Supreme Court ruled that claims to a diagnostic method were invalid where the claims were directed to using a particular level of 6-thioguanine as an indicator of whether a drug dose should be modified. The Court determined that the claims did not do “significantly more than simply describe … natural relations.” Mayo Collaborative Services v. Prometheus Laboratories, Inc., 566 U.S. ___, 132 S.Ct. 1289 (2012).
- In June 2013, the Court analyzed claims to isolated genomic DNA segments associated with the BRCA genes and methods of diagnosing cancer propensity by detecting mutations in the genetic sequences. The Court held that isolating a genomic DNA segment was insufficient to provide patent eligibility. The Court reasoned that, while Myriad “found an important and useful gene, … separating that gene from its surrounding genetic material is not an act of invention.” However, cDNA, which lacks the non-coding regions of genomic DNA, was held to be patentable, as it is not naturally occurring. Ass’n for Molecular Pathology v. Myriad Genetics, Inc., 569 U.S. ___, 133 S.Ct. 2107 (2013).
- In June 2014, the Supreme Court considered four patents with claims directed to electronic methods, computer systems, and program code for mitigating settlement risk. The Court declined to “labor to delimit the precise contours” of the abstract-idea exception, while concluding that these claims were “squarely within [the exception’s] realm” and were patent ineligible. Alice Corp. v. CLS Bank Int’l, 134 S.Ct. 2347 (2014).
Taken together, these cases have called into question the validity of many issued claims and the patentability of claims in many pending applications. The United States Patent and Trademark Office (USPTO) has developed multiple sets of guidelines to address and implement the new case law generated by these Supreme Court rulings. The guidelines represent the USPTO’s attempt to provide clarity to its examiners and to applicants despite the flexibility or vagueness (depending on one’s perspective) of the Court’s decisions. The challenge before the USPTO is to translate the decisions into rules that can be consistently applied by each of thousands of examiners. Mayo prompted the Interim Laws-of-Nature Guidelines in July 2012 (“the Mayo guidelines”); Myriad prompted the Guidelines responsive to Myriad and Mayo in March 2014 (“the Myriad guidelines”); Alice prompted “Preliminary” Guidelines in June 2014; and, most recently, in December 2014, the USPTO provided “Interim” Guidelines to further address Alice, Myriad, and Mayo.
Thus, within the past three years, we have witnessed no less than three Supreme Court decisions and four sets of USPTO guidelines focused on subject matter eligibility. Hypotheses as to what effect various decisions and guidelines have had on patent examination have been plentiful, but there are few studies that have empirically addressed the issue. Accordingly, in an effort to determine the effect of the Supreme Court decisions and the various guidelines on how examiners evaluate patentable subject matter under § 101 in Technology Center 1600 (TC 1600), we collected examination data at various time points during this busy three-year period.
[Bio-Pharma]
Data Collection
We first selected six time periods of interest. These time periods are summarized in Table 1. For each of these time periods, we identified USPTO actions issued between the 14th and 28th of the identified month using LexisNexis Patent AdvisorSM. Further using this technology, we determined whether each action was an allowance or a rejection, and, if the latter, the types of rejections in the office action.
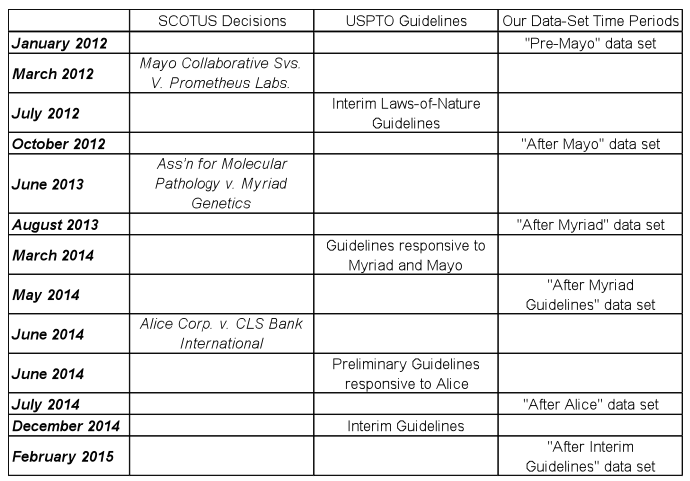
Table 1. Relative timing of select decisions from the Supreme Court, issuance of new USPTO guidelines, and time periods for which office-action data was collected for the present study.
We separated data from Technology Center (TC) 1600 to identify examination trends across the biotechnology and organic chemistry art units. TC-1600 data were segregated into art unit groups as shown in Table 2. Table 2 further identifies the specific art unit groups and the technologies associated with each. Data as described below were then generated.
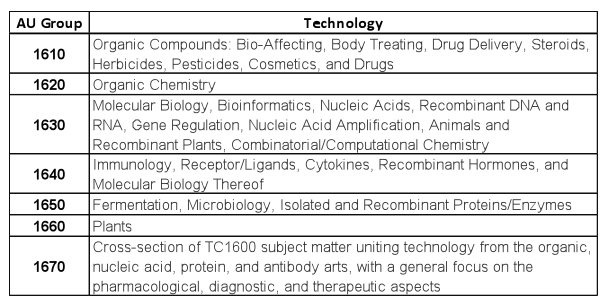
Table 2. Technologies associated with each art unit group in the biotechnology and organic chemistry TC 1600.
Patent-Eligibility Rejections: Substantially more Prevalent in Select Art Unit Groups
For each office action issued during one of the six defined time periods, we identified, with a custom query executed by LexisNexis Patent AdvisorSM, whether the office action included a rejection under § 101. We assumed that most, if not all, of the 101 rejections included assertions that one or more claims lacked patentable subject matter.
Figure 1 shows how the prevalence of 101 rejection varies across art unit groups and time periods. The 101 prevalence was rather constant across time periods in the organic compounds and organic chemistry art unit groups 1610 and 1620. Further, no consistent trend was observed in the plant art group 1660. Meanwhile, the art unit groups focusing on molecular biology, immunology and protein chemistry technologies (art unit groups 1630, 1640, 1650 and 1670) showed steady increases in 101 prevalence. Across all of these art units, the percentage of office actions with a 101 rejection tripled from the January 2012 Pre-Mayo time period to the more recent February 2015 After Interim Guidelines time period. Specifically, the probability of receiving a rejection under § 101 rose from 11.6% in January 2012 to 32.5% in February 2015. The sharpest increase occurred after the Myriad Guidelines (as the probability jumped from 16.3% in August 2013 to 26.1% in May 2014), and, although the Interim Guidelines were generally viewed as more reasonable than the Myriad Guidelines, the occurrence of rejections under § 101 was higher after the Interim Guidelines (32.5%) than after the Myriad guidelines (26.1%).
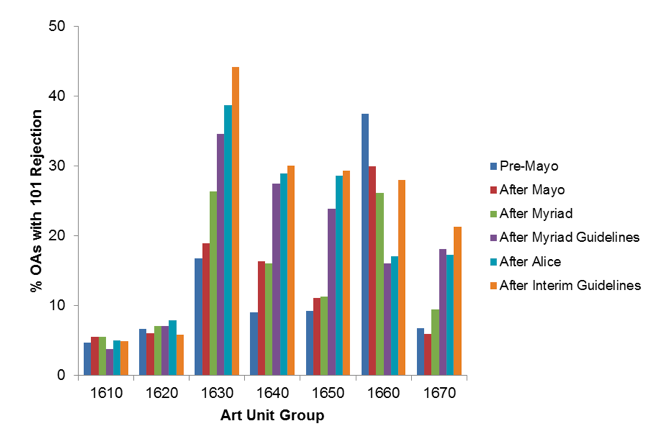
Figure 1. Prevalence of 101 rejections in office actions. For each non-final and final office action issued during the indicated time period (as described above), LexisNexis Patent AdvisorSM data indicated whether the action included a 101 rejection. Percentages of office actions issued during the time period that included such a rejection were calculated for each art unit group in TC 1600.
Also, art unit group 1630 showed a more substantial effect after the Myriad decision than did art unit groups 1640, 1650, or 1670. These differences may reflect the fact that the Myriad decision was a narrow ruling related to genomic DNA, whereas the Myriad Guidelines reached beyond DNA and included naturally occurring proteins. Art unit group 1630, which evaluates claims to isolated DNA would have been immediately affected by Myriad, whereas art units 1640, 1650, and 1670, which evaluate more claims directed to naturally occurring proteins, delayed their response to Myriad until instructed to do so by the Guidelines.
Effects on Prosecution Outcomes
Several strategies exist for responding to a rejection under § 101. An applicant may be able to amend one or more claims or to argue against the rejection (to the examiner or to the appeal board) so as to overcome the rejection. Alternatively, an applicant may believe that it will be impossible to overcome the rejection or that the prosecution costs or risks outweigh the patent incentive. In these instances, an applicant may choose to abandon prosecution of a patent application.
To investigate how the rejections under § 101 affect prosecution, we identified the percentage of actions that were notices of allowances (as opposed to office actions). Figure 2 shows these percentages for each of our defined time periods by TC 1600 art unit group. Interestingly, we do not observe strong trends in allowance rates across the defined time periods. Art unit groups 1610 and 1620, two of the groups showing little change in the prevalence of rejections under § 101, show a relatively steady increase in allowance rates, whereas art unit groups 1630, 1640, and 1650 showed allowance-probability variability but no upward trends in allowance rates.[1]
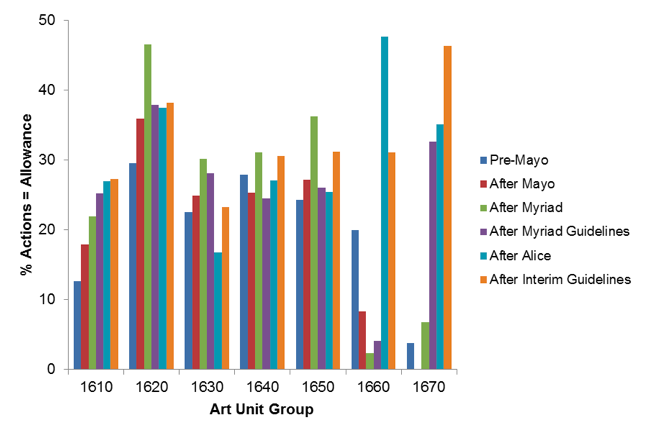
Figure 2. Prevalence of notices of allowances. Each action issued during the indicated time period, was identified (via LexisNexis Patent AdvisorSM) as a notice of allowance or an office action. The y-axis shows the percentage of actions that were notices of allowance as opposed to non-final or final office actions. (Notices of abandonments were not considered in this analysis, due to the applicant control of an abandonment decision.)
However, securing a patent does appear to require additional effort in recent time periods. Figure 3 shows the average number of office actions issued prior to issuance of notices of allowance in an indicated time period. Across the 1630, 1640 1650 and 1670 biotechnology art units, the average office-action count rose 25% from 2.0 in the January 2012 Pre-Mayo time period to 2.5 in the February 2015 After Interim Guidelines time period. Art unit group 1630, which evaluates claims related to molecular biology and DNA, among other things, shows the highest number of office actions before allowance than any other art unit group shown.
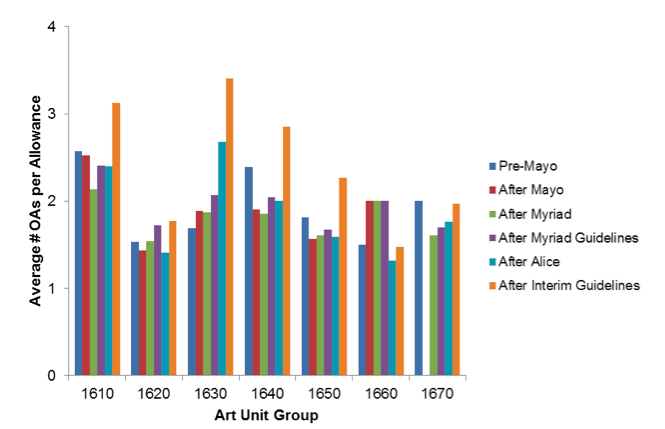
Figure 3. Office actions per allowance. For each notice of allowance issued during the indicated time period, LexisNexis Patent AdvisorSM data identified a number of final or non-final office actions issued prior to the allowance. The y-values identify the average of these counts for a particular art unit group and time period.
PTAB Indications of Applicant Caution
One may posit: is the striking increased prevalence of 101 rejections in biotechnology area justified in view of the new case law or does it represent an overreaction by examiners? If the latter, one may expect that an increasing number of applicants would challenge 101 rejections by appealing these rejections and that the Patent Trial and Appeal Board (PTAB) would frequently reverse the rejections.
We therefore performed searches within the PTAB ex parte appeal database for decisions between January of 2012 to July of 2015 in TC 1600 that involved an appeal related to a rejection of claims based upon a purported lack of statutory subject matter under 35 U.S.C. § 101. Interestingly, we found that very few TC 1600 PTAB decisions involved a challenged or newly raised 101 rejection. Specifically, for this decision time period, we identified only one decision involving patent-eligibility issues, and that was in the context of an interference proceeding.
To further explore whether applicants were more or less likely to appeal given the various rulings and guidelines, we used LexisNexis Patent AdvisorSM to identify the number of appeal briefs filed in TC 1600 and other Technology Centers since January 1, 2012.
As shown in Figure 4A, applicants in TC 1600 are generally less likely to appeal than applicants in other Technology Centers. For example, the number of briefs filed in the beginning of 2012 in TC 1600 was only 57% that of the average across other technology centers during that time period, and the percentage rose only slightly (to 59%) for the late 2014 time period.
For TC 1600, we further segregated the briefs by art-unit group and six-month time periods. (See Figure 4B.) Across the 1630, 1640, 1650, and 1670 art unit groups, which were the groups showing a marked increase in 101 rejections, there were 339 appeal briefs filed in the first half of 2012 and only 206 filed in the last half of 2014 – a 40% drop.
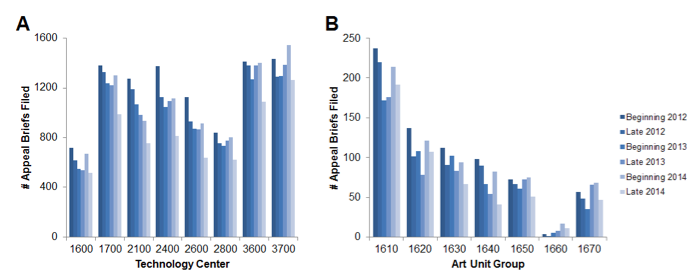
Figure 4. Number of appeal briefs filed (A) per technology center; and (B) per art unit group within Technology Center 1600. The y-value shows the number of appeal briefs filed within the designated time period within each identified technology center art unit group. Time periods identified as being in the “beginning” of the year span from January 1 to June 30; “late” time periods span from July 1 to December 31.
We propose that – among other considerations – the rapid changes in case law and USPTO interpretation of 35 U.S.C. § 101 is providing applicants with a disincentive to aggressively challenge rejections and to seek expedited resolution of issues. Rather, applicants may be taking the position that methodically and iteratively addressing rejections may be beneficial in achieving an allowance or in narrowing the issues for appeal. For example, concentrating on prior-art and § 112 rejections while postponing § 101 arguments may allow an applicant to gain further clarity as to how § 101 is being applied. Applicants may be hoping we have reached the nadir with regard to rejections under § 101 and may prefer to delay appeals until additional case law improves their chances of success on appeal of a rejection under § 101.
Conclusions
There is good news – the USPTO continues to issue patents related to biotechnology and organic chemistry inventions despite the Supreme Court rulings and USPTO guidelines implementing the ruling related to the scope of patentable subject matter. Although the sky has not fallen, applicants must expect more rejections under 35 U.S.C. § 101 and must budget for more office actions before receiving an allowance from TC 1600. Furthermore, applicants can expect these challenges in several art unit groups, particularly in art unit group 1630. As a matter of strategy, if a rapid allowance is sought, applicants should carefully draft applications and claims to comply with the Interim Guidelines and utilize options to speed prosecution. Because of the uncertainty in the relevant case law and its rapidly evolving nature, applicants should consider whether to appeal intractable rejections under § 101. Further analysis is necessary to determine whether appeals of rejections under § 101 by TC 1600 are successful.
__________
[1] The count of total actions issued across all time periods for art unit group 1660 was low (388; while the count exceeded 2000 for each other TC 1600 group). The limited sample size likely contributes to the variability observed for this art unit group.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
One comment so far.
T2
July 13, 2015 08:35 amA thoughtful and interesting analysis; thank you. We have needed to reduce scope in many cases, such as claiming a DNA sequence within an appropriate vector. The Myriad decision appears to affect composition claims related to other isolated natural products as well- e.g., proteins, lipids, carbohydrates. In the meantime, there may be increased interest in the exclusivity offered through regulatory agencies which could become even more important in light of the inter partes review system under the AIA.