 On Thursday, August 18th, Irish pharmaceutical developer Horizon Pharma PLC (NASDAQ:HZNP) filed an action for patent infringement against two parties: Actavis, a subsidiary of fellow Dublin-based drugmaker Allergan PLC (NYSE:AGN); and Mumbai, India-based Lupin Pharmaceuticals (NSE:LUPIN). Both actions were brought in response to the filing of Abbreviated New Drug Applications (ANDAs) by both defendants for a generic version of Pennsaid, a topical nonsteroidal anti-inflammatory drug (NSAID) marketed in the U.S. by Horizon. Both cases were filed in the U.S. District Court for the District of New Jersey (D.N.J.).
On Thursday, August 18th, Irish pharmaceutical developer Horizon Pharma PLC (NASDAQ:HZNP) filed an action for patent infringement against two parties: Actavis, a subsidiary of fellow Dublin-based drugmaker Allergan PLC (NYSE:AGN); and Mumbai, India-based Lupin Pharmaceuticals (NSE:LUPIN). Both actions were brought in response to the filing of Abbreviated New Drug Applications (ANDAs) by both defendants for a generic version of Pennsaid, a topical nonsteroidal anti-inflammatory drug (NSAID) marketed in the U.S. by Horizon. Both cases were filed in the U.S. District Court for the District of New Jersey (D.N.J.).
Horizon is attempting to assert its rights on four patents related to the production of Pennsaid. The patents-in-suit for both cases are:
- U.S. Patent No. 9339551, which is titled Diclofenac Topical Formulation. It claims a method for treating pain by applying a gel formulation of diclofenac sodium to someone suffering from chronic joint disease such as osteoarthritis.
- U.S. Patent No. 9339552, same title as above. This patent protects the topical formulation including diclofenac sodium which has a better drying time, increased transdermal flux and greater pharmacokinetic absorption than previous compositions.
- U.S. Patent No. 9375412, entitled Treatment of Pain with Topical Diclofenac. This patent discloses a method for applying topical agents to a knee of a patient with pain by applying a topical diclofenac formulation to a patient’s knee, waiting for the area to dry, applying a sunscreen or insect repellant to the treatment area and counseling the patient to avoid taking unprescribed acetaminophen while taking the topical diclofenac formulation.
- U.S. Patent No. 9370501, same title as the ‘412 patent. It protects a method for applying topical agents to a knee of a patient with pain using a different topical diclofenac formulation than the ‘412 patent which includes a thickening agent and a polyhydric alcohol.
The patents-in-suit protect the solution and treatment applications of Pennsaid, a topical NSAID solution of which two percent of its weight is comprised of diclofenac sodium. Horizon Pharma had received notification letters in recent weeks that both Actavis and Lupin had filed ANDAs with the U.S. Food and Drug Administration (FDA) for generic versions of 2% diclofenac sodium topical solution. Both Lupin and Actavis contended that the ‘551 and ‘552 patents were invalid and unenforceable. Horizon added the ‘412 and ‘501 patents to its lawsuit.
If Lupin or Actavis gain regulatory approval for their generic formulations, Horizon is seeking monetary relief together with interest from the defendants. Horizon is also asking the court to exercise powers outlined by 35 U.S.C. §271(e)(4)(a) to delay the effective date of any FDA approval of either ANDA until after the infringed patents have expired. Horizon contends that the earliest that the ‘551 and ‘552 patents will expire is October 17th, 2027, and the earliest that the ‘412 and ‘501 patents will expire is July 10th, 2029.
In some cases, a patent infringement lawsuit filed in response to an ANDA for a generic pharmaceutical can result in a licensing agreement settled out of court. In May 2014, Actavis entered into a licensing agreement with Canadian generic drugmaker Valeant Pharmaceuticals (NYSE:VRX) to market a generic version of Valeant’s Acanya, a topical treatment for acne vulgaris. It’s possible that Actavis and Lupin could reach similar licensing agreements to settle patent infringement suits related to their ANDA filings.
The use of paragraph IV patent certifications in both the Lupin and Actavis ANDA filings for generic 2% Pennsaid were cited in Horizon’s lawsuits. Paragraph IV certifications are made by generic drugmakers when filing ANDAs when trying to assert that any patents covering brand name drugs are either invalid or will not be infringed by the generic. This is one of four types of certifications that must be made by an ANDA under the terms of the Drug Price Competition and Patent Term Restoration Act, also known as the Hatch-Waxman Act. Other certifications which can be made in an ANDA include paragraph I certifications, when no patent information on a brand name drug has been submitted to the FDA; paragraph II certifications, when the patent has expired; or paragraph III certifications, which acknowledge the date on which a patent will expire, allowing the ANDA to be accepted. According to a table published by the FDA, the earliest paragraph IV certification for a generic version of Pennsaid topical solution was filed July 11th, 2012.
Horizon Pharma purchased the U.S. sales and marketing rights to Pennsaid in October 2014 from the drug’s developer, Nuvo Pharmaceuticals (TSE:NRI) of Mississauga, Ontario, Canada. Horizon purchased those rights for $45 million while Nuvo retains the rights to Pennsaid outside of the U.S. Interestingly, the press release for that 2014 sale noted that there are six U.S. patents protecting Pennsaid, not just the four patents asserted in the Actavis and Lupin cases. Horizon’s most recent quarterly earnings report noted that the company had a binding purchase agreement in early June with Nuvo, which manufactures and supplies Pennsaid to Horizon, for $4.4 million worth of Pennsaid through September of this year. Net sales of Pennsaid for the three months ending June 30th were $72.7 million, a 147 percent increase in the $29.4 million in net sales seen during the three months ending June 30th, 2016, making Pennsaid the top seller for Horizon during the recent quarter. $26.7 million worth of net sales growth was attributed to higher net pricing and another $16.5 million was gained through higher prescription volumes.
That recent earnings report also identified other companies filing ANDAs for generic Pennsaid which Horizon Pharma is trying to challenge in court. Horizon has also filed suit against Paddock Laboratories, a subsidiary of Allegan, MI-based pharmaceutical developer Perrigo (NYSE:PRGO); Taro Pharmaceuticals (NYSE:TARO) of Hawthorne, NY; Teligent Inc. (NASDAQ:TLGT) of Buena, NJ; and Amneal Pharmaceuticals LLC of Bridgewater, NJ. Each of these actions arose from paragraph IV certifications and in each case, the litigation was settled out of court with Horizon granting non-exclusive licenses to manufacture and commercialize generic Pennsaid which give Horizon a percentage of the generic’s net sales.
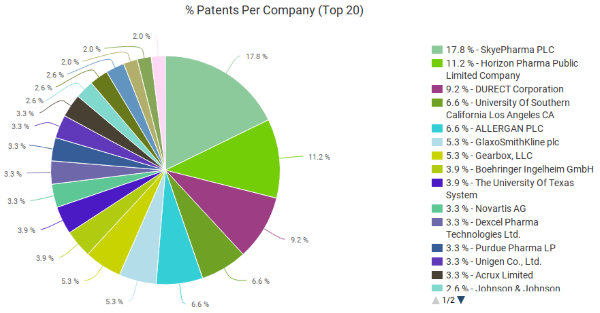
Topical analgesics do not account for most of the sales in the pain relief treatment market, only accounting for $40 million in sales as of 2007 including diclofenac and other treatments. Most of the market is dominated by orally administered analgesics. However, there are a number of companies holding patents related to topical NSAIDs; the patent portfolio analysis tools available through Innography identifies 357 U.S. patents when searching for topical NSAIDs. As the pie chart (below) will show readers, the largest patent portfolio in this field belongs to Britain’s SkyePharma PLC (LON:SKP), which holds 17.8 percent of the patents in this field. Horizon Pharma was in second place holding 11.2 percent of this patent market and Durect Corporation (NASDAQ:DRRX) of Cupertino, CA, sits in third place with 9.2 percent of the U.S. patents in this field.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[[Advertisement]]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-2024-banner-938x313-1.jpeg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
No comments yet.