On July 17, 2017, the Court of Appeals for the Federal Circuit reversed a district court decision that declared several claims of U.S. Patent No. 6,713,446 (“the ‘446 Patent”) as invalid as obvious. This case concerned Millennium Pharmaceuticals in its multiple lawsuits against multiple defendants in ANDA litigation.
Factual Background
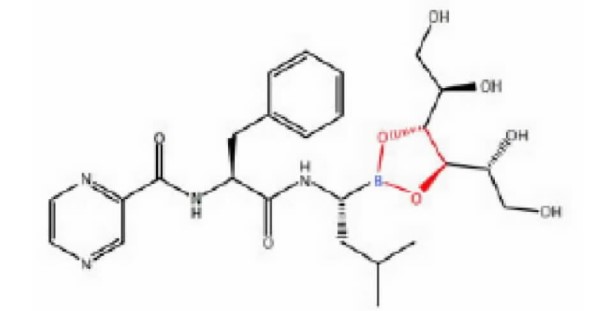
Millennium Pharmaceuticals, Inc., as an exclusive licensee of the ‘446 patent from the United States Government, developed a drug for treating multiple myeloma and mantle cell lymphoma under the brand name Velcade®. Millennium Pharmaceuticals, Inc. v. Sandoz, Inc., Appeal no. 2015-2066, 2017 WL 3013204 at *1 (Fed. Cir. July 17, 2017). In particular, the chemical compound that is the subject of the ‘446 patent is D-mannitol N-(2-pyrazine) carbonyl-L-phenylalanine-L-leucine boronate, a boronate ester of bortezomib and D-mannitol (a hydroxyl compound), where the lyophilized (i.e., “freeze-dried”) compound is claimed in Claim 20. Id. The foregoing compound is shown with Millennium’s highlight of the bonds between the bortezomib moiety and the D-mannitol moiety:
Id.
In addition, there are claims to a lyophilized cake, method of preparation of the new compound and its reconstitution of the lyophilized mixture with a pharmaceutically acceptable carrier. Id. at *1–2.
It was known in the art that the base compound bortezomib had chemotherapeutic effects but never was approved by the FDA due to its instability, rapid degradation in liquid formulations and general insolubility. Millennium Pharmaceuticals, 2017 WL 3013204, at *2. The inventor tried to develop twenty-five liquid formulations but failed in overcoming the stability and solubility problems that plagued researchers in the field. Id.
Finally, the inventor and associated researchers turned to using lyophilization (i.e., freeze-drying). Id. Such a process in the presence of mannitol, a known bulking agent was utilized in order to prepare a finalized formulation and was not intended to change the chemical structure of the active pharmaceutical ingredient. Id. Unexpectedly, a new chemical ester compound was formed during the process and had substantial improvement in dissolution and stability. For instance, once the claimed ester compound was administered into a patient, the ester compound “converts to or releases the active pharmaceutical ingredient upon administration to a patient.” Id.
Although the subject case has been remanded in order for providing defendants the opportunity to present their case for finding obviousness with respect to claims of the ‘446 patent, patent practitioners, inventors and corporate executives can review the following five important lessons from the holding of the case.
1. A teaching or suggestion in the prior art that the intended result would occur is needed in formulating a finding of obviousness even, if at first glance, the process used may be routine
As the following shows, it remains important to find a teaching or suggestion in the prior art that a given result would occur even in seemingly routine processes because the intended results may be surprising and unexpected. First, there was no teaching or suggestion in the prior art that a new chemical compound would be created during lyophilization, a process not intended to change chemical properties, and solve the problems that inhibited development of bortezomib in oncology. Millennium Pharmaceuticals, Inc. v. Sandoz, Inc., 2017 WL 3013204 at *2. Secondly, there was no teaching or suggestion in the prior art reference of unexpected properties of dramatically improved stability, solubility and dissociability of the newly created compound over the base compound, which researchers have known to be plagued with problems. Id. at *2 and *8. Thus, it is important for patent attorneys to discuss with the inventors on recognizing whether certain experimental results are unexpected in relation to the expectations of the prior art.
2. General motivation for a person of ordinary skill insufficient to find obviousness in many cases
As the following shows, a general motivation to use a known process, in certain cases, can be insufficient to find a particular claimed chemical compound produced using a known process as obvious. Bortezomib is a lead compound for consideration in an obviousness determination on whether a person of ordinary skill would have the reason or motivation to modify it to make a claimed compound with a reasonable expectation of success. Millennium Pharmaceuticals, 2017 WL 3013204, at *4 (citations omitted by author). However, a specific motivation to modify bortezomib using the known pharmaceutical process with a reasonable expectation of success was lacking in this case. For instance, although a general motivation to modify pharmaceutical products using lyophilization and a process of adding bulking agents was present, no specific motivation to modify bortezomib was shown in this case. For instance, there was no teaching or suggestion in the references to produce the claimed mannitol ester having the unexpected properties of improved stability and solubility by specifically lyophilizing bortezomib and using mannitol as a bulking agent for bortezomib. Id. at *5. In fact, stabilization of a particular compound by ester formation was unpredictable, because such process was compound-specific, and a person of ordinary skill expected that an ester would block a portion of the bortezomib molecule, rather than expecting that the ester compound to disassociate in the bloodstream effectively. Id. at *6. Thus, it is important for patent attorneys and inventors, when possible, to recognize why a specific motivation to modify a lead compound with a reasonable expectation of success is lacking. This is extremely important if they want to successfully overcome rejections in the USPTO and succeed in litigation in the future.
3. Inherency in the context of obviousness still requires that the claimed invention be expected by a person of ordinary skill
Inherent obviousness cannot be based on what the inventor thought, and, in addition, the results in a particular case may not be inherently obvious depending on what was expected by a person of ordinary skill. The court pointed out “’the mere fact that a certain thing may result from a given set of circumstances is not sufficient’ to render the results inherent.” Millennium Pharmaceuticals, 2017 WL 3013204, at *6 (citations omitted by author). The court also held that it is never appropriate to consider “what the inventor intended when the experiment was performed,” even though Millennium “conceded as a matter of law that the ester is a ‘natural result’ of freeze-drying bortezomib with mannitol.” Id. Thus, hindsight reasoning should never be applied and, obviousness is “measured objectively in light of the prior art, as viewed by a person of ordinary skill in the invention.” Id. at *7. In this case, “no expert * * * foresaw or expected or would have intended, the reaction between bortezomib and mannitol, or that the resulting ester would have the long-sought properties and advantages.” Id.
Incidentally, more recently, in Honeywell International Inc. v. Mexichem, the Federal Circuit reaffirmed the foregoing principle on focusing on what a person of ordinary skill would have expected. Honeywell International Inc. v. Mexichem Appeal No. 2016-1996, 2017-WL3254943 (Fed. Cir. Aug. 1, 2017) at *5. In a case involving a Honeywell patent claiming a heat transfer composition claiming a combination of a 1,1,1,2-tetrafluropropene (“HFO-1234yf”) and a polyethylene glycol (“PAG”) lubricant, the court held that a patent owner need only establish that the results would have been unexpected to one of ordinary skill at the time of the invention or “much greater than would have been predicted.” Honeywell International Inc. v. Mexichem, 2017 WL 3254943 at *5 (citations omitted by author). The court also emphatically asserted:
We have previously stated that the use of inherency in the context of obviousness must be carefully circumscribed because “[t]hat which may be inherent is not necessarily known” and that which is unknown cannot be obvious. [Id. at *4] [citations omitted by author.]
The court went on to state that “[w]hat is important regarding properties that may be inherent, but unknown, is whether they are unexpected” (emphasis added). Id. The court pointed out that “[a]ll properties of a composition are inherent in that composition, but unexpected properties may cause what may appear to be an obvious composition to be nonobvious” (emphasis added). Id.
In addition, examiner needs to show that one of ordinary skill would have had a motivation to combine the references with a reasonable expectation of success. Id. at *5 (citations omitted by author.) The court’s reasoning seems to suggest that the foregoing principle hold true when even though, generally, stability is an inherent property of a refrigerant/lubricant pair, stability may not be a property possessed by the specific combination of the HFO refrigerant and the PAG lubricant. The evidence on the record showed that there was unexpected stability of the claimed HFO-1234yf with PAG lubricants and a person of ordinary skill would have expected that combining reactive and unstable HFO refrigerants and unstable PAG lubricant would, in fact, result in a degradation reaction due to peroxide formation. Id. at *2. Thus, it is important for patent attorneys and inventors to recognize in situations even when conventional processes are used, such results, although inherent, may be unknown, and therefore, unexpected!
Now turning back to the Millennium Pharmaceuticals case, objective evidence of traversing an obviousness finding or what is also known in the patent law as “secondary considerations” include unexpected results and long-felt but unsolved need. The following will discuss the foregoing factors mentioned by the court in Millennium Pharmaceuticals.
4. Unexpected results are to be compared with what was known in the prior art
When unexpected results are used as evidence of non-obviousness, the results must be shown to be unexpected when compared with the closest prior art. For instance, the district court was incorrect in requiring Millennium to compare improve stability, solubility, and dissolution properties of the lyophilized mannitol ester of boretezomib with a glycerol bortezomib ester mentioned in U.S. Patent No. 5,780,454 (the “Adams patent”) referenced by defendant Sandoz, rather than boretezomib. Millennium Pharmaceuticals, 2017 WL 3013204, at *8. The court’s reasoning seems to follow from several factual determinations. The Adams patent generically identified glycerol as one amongst a list of ten compounds for boronate esters, rather than specifically disclosing or actually identifying the glycerol ester in the Adams Patent. Id. Moreover, the Adams Patent did not disclose stability or solubility of any ester compound. Id. Accordingly, Millennium was not required to create the glycerol ester, where the product had not been created in the prior art. (Id. at * 8)(citations omitted by author). Thus, it is important for patent attorneys and inventors to compare only unexpected results with only prior art that is known.
5. Long-felt but unsolved need met when prior unmet demand achieved by patented product
Long-felt but unsolved need is met when (1) a demand existed for the patented invention; and (2) others tried but failed to satisfy that demand. Millennium Pharmaceuticals, 2017 WL 3013204, at *8, (citations omitted by author). The district court’s finding was in error, because there was a long-felt need for a product to treat multiple myeloma, because prior treatments gave poor remission and low survival rates, and due to the unavailability of bortezomib as a viable commercial product. Id. at *9. The D-mannitol ester was responsible for Velcade®’s successful results and provided required solubility and stability. Id. Thus, it is important for patent attorneys and inventors to recognize that a long-felt but unsolved need can be met when a claimed compound satisfies an unmet demand.
As the foregoing shows, it remains even more important for inventors, during the patent specification drafting process, to discuss with their patent attorneys any finding of unexpected results when comparing such results with the closet prior art. This is not only useful when drafting a patent specification in order to overcome prior art rejections at the USPTO but also can assist the inventors in litigation in the future.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/03/IP-Copilot-Apr-16-2024-sidebar-700x500-scaled-1.jpeg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
2 comments so far.
Jay Pattumudi
August 11, 2017 08:53 amThank for your comments and I wish you best of luck in securing a position!
Many examiners don’t know the law of obviousness as applied in the pharma context. The cases mentioned in my article will assist them in gaining a proper understanding of the legal standard for inherency in the context of obviousness.
BV
August 10, 2017 09:53 amShould I ever get a job again, I will cite this in rebuttal of the examiner’s obviousness rejection.
I will then anxiously await the response from the examiner where he, she, they, it, etc bsically says “I don’t care about your patent law!”