 On Thursday, April 20th, the Court of Appeals for the Federal Circuit issued a decision in Medinol Ltd. v. Cordis Corporation et. al. which vacated and remanded a lower court’s ruling that claims of patent infringement alleged by Israeli pharmaceutical firm Medinol were barred by the equitable defense of laches. The Federal Circuit’s decision comes after the U.S. Supreme Court overturned the Federal Circuit’s previous precedence on laches as an equitable defense in SCA Hygiene Products v. First Quality Baby Products, decided last year. The case was decided by a panel consisting of Circuit Judges Timothy Dyk, Jimmie Reyna and Kara Stoll.
On Thursday, April 20th, the Court of Appeals for the Federal Circuit issued a decision in Medinol Ltd. v. Cordis Corporation et. al. which vacated and remanded a lower court’s ruling that claims of patent infringement alleged by Israeli pharmaceutical firm Medinol were barred by the equitable defense of laches. The Federal Circuit’s decision comes after the U.S. Supreme Court overturned the Federal Circuit’s previous precedence on laches as an equitable defense in SCA Hygiene Products v. First Quality Baby Products, decided last year. The case was decided by a panel consisting of Circuit Judges Timothy Dyk, Jimmie Reyna and Kara Stoll.
Medinol first filed suit against both Cordis and American consumer goods giant Johnson & Johnson in the Southern District of New York back in March 2013. In the complaint, Medinol asserted a series of four U.S. patents covering technologies related to coronary stents, including:
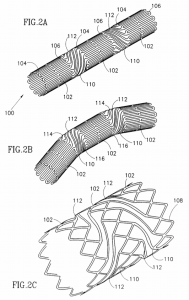 U.S. Patent No. 5980552, titled Articulated Stent. Issued in November 1999, it covers a connector for connecting adjacent areas of substantially tubular and substantially rigid segments of an articulated stent which addressed issues posed by non-uniform and undesirable stresses of conventional articulated stents on blood vessels.
U.S. Patent No. 5980552, titled Articulated Stent. Issued in November 1999, it covers a connector for connecting adjacent areas of substantially tubular and substantially rigid segments of an articulated stent which addressed issues posed by non-uniform and undesirable stresses of conventional articulated stents on blood vessels.- U.S. Patent No. 6875228, same title as the ‘552 patent. Issued in April 2005, it claims an expandable stent at least two substantially longitudinally rigid segments, each segment having a plurality of U- or V-shaped segments in both the expanded and unexpanded configuration, each of the rigid segments presenting a substantially cylindrical structure; and then a flexible connector having a plurality of flexible links connecting the U- or V-shaped segments of adjacent rigid segments
Medinol had alleged that Cordis and Johnson & Johnson had willfully infringed upon the patents-in-suit by engaging in the sale of Cypher and Cypher Select stents for patients with heart disease. Cypher stent sales were discontinued by Cordis in 2011. Defendants Cordis and J&J raised laches as a defense and, after a bench trial, U.S. District Judge Shira Scheindlin issued an opinion and order in March 2014 which dismissed Medinol’s patent complaint. The opinion includes various findings of fact regarding the relationship between the plaintiffs and defendants going back to April 2000, when Medinol and its licensee Boston Scientific first filed suit against Cordis over stent products marketed by that entity. In that case, Medinol filed patent infringement claims against Cordis over that firm’s marketing of BX Velocity stents, the precursor to the Cypher stents and “the real product at issue” in the 2013 suit, asserting a series of patents which covered uniformly-flexible stents, not articulated stents covered by the patents from the more recent lawsuit.
Judge Scheindlin “reasonably inferred” that Medinol did not believe that the patents asserted in its 2013 case against Cordis, which had never been licensed to a medical devices company, were as strong as the patents from the 2000 suit, which had been licensed. The patents asserted by Medinol in 2000 were continuations in part from U.S. Patent No. 9449373, titled Articulated Stent and the parent application of the patents asserted in the 2013 case. The 2000 case resulted in findings of patent invalidity which were upheld by the Court of Appeals for the Federal Circuit.
In testimony regarding the 2013 lawsuit, Medinol’s chairman said that he didn’t realize that the BX Velocity stents infringed on the articulated stent patents until 2005, but Judge Scheindlin didn’t find that testimony to be credible as Cordis itself had argued that the BX Velocity stent achieved flexibility through articulation points, not uniform flexibility. “This was obvious from Cordis’s advertising of the BX Velocity and from the arguments it advanced during the August 2000 preliminary injunction hearing and the September 2001 trial,” Judge Scheindlin’s opinion reads.
In 2006, around the same time that Medinol retained counsel to explore the possibility of a patent infringement suit on the articulated stent patents, Medinol actually entered into licensing talks with Cordis after the license with Boston Scientific ended and Cordis couldn’t complete a merger to bolster its stent business. As part of the negotiations, Medinol and Cordis entered into an agreement in the summer of 2006 to toll the applicable statute of limitations for any cause of action between the parties until July 2007. About that time, Medinol and Cordis did enter into a distribution agreement which gave Medinol a high royalty rate for supplying bare-metal stents while allowing Cordis to sell competing stents as well and this distribution agreement was extended to 2014. The distribution agreement ended all existing patent litigation and tolled the statute of limitations for applicable claims between the parties until July 2008. While the distribution agreement ended in 2011 when Cordis chose to leave the stent market, Medinol and Cordis continued to have business discussions about tolling claims despite the fact that Medinol did not disclose the potential lawsuit on the articulated stent patents.
“Even if the period of maximum delay begins with the issuance of each individual patent, the delay for each patent is still significantly longer than the presumptively unreasonable six years,” Judge Scheindlin wrote. The court found that Medinol’s understanding of the potential claim on the articulated stent patents extended back to the 1999 release of the BX Velocity stents; the patents weren’t asserted at that time because Medinol “may have been forced to take conflicting positions in the same lawsuit about whether the BX Velocity is or is not uniformly flexible.” Any excusable delay in bringing the suit ended in October 2004 when the U.S. Supreme Court declined to issue a writ of certiorari in the first case between Medinol and Cordis. Cordis was further able to prove economic prejudice to warrant the application of laches as the court found that Cordis would not have entered into the distribution agreement with Medinol if there was the potential of litigation regarding Medinol’s articulated stent patents.
In a pretrial memorandum of law filed by Medinol, the plaintiff reserved its right to argue that laches should not be applied to bar claims of patent infringement based on the outcome of Petrella v. Metro-Goldwyn Mayer, a case taken up by the Supreme Court on the question of whether laches can bar claims in copyright cases. A few months after Judge Scheindlin issued the order dismissing Medinol’s claims on Cordis’ laches defense, Medinol filed a request for relief from judgment under Federal Rule of Civil Procedure 60(b)(6). Medinol reasoned that the Supreme Court’s finding in Petrella that laches did not bar copyright claims similarly applied to Medinol’s patent infringement claims and that the Petrella decision constituted an “extraordinary circumstance” that justified vacature of the finding for Cordis based on the laches defense. In September 2014, Judge Scheindlin issued an order denying Medinol’s motion to vacate the judgment under Rule 60(b)(6), finding that Petrella only applied to claims under the Copyright Act and didn’t disturb the framework regarding laches defenses in patent cases set by the Federal Circuit’s 1992 decision in A.C. Aukerman Co. v. R.L. Chaides Construction.
Medinol appealed the ruling on its Rule 60(b)(6) motion to the Federal Circuit and the appellate court initially held the appeal in abeyance as it came during its en banc rehearing period for SCA Hygiene v. First Quality Baby Products. The Federal Circuit would end up upholding laches as a defense in patent cases when it decided SCA Hygiene in September 2015. After that decision, Medinol and Cordis both filed for summary affirmance in light of SCA Hygiene and Medinol filed a writ for certiorari with the U.S. Supreme Court. SCOTUS held Medinol’s petition before reversing the Federal Circuit’s SCA Hygiene holding in October 2016, holding that laches cannot be as a defense for damages against patent infringement if the infringement claims are brought within the six-year limitations period under 35 U.S.C. § 286.
Following its decision in SCA Hygiene, SCOTUS then granted writ to Medinol, vacated the Federal Circuit’s judgment and remanded the case for further proceedings. Because SCOTUS’ finding in SCA Hygiene overruled the Federal Circuit’s precedent from A.C. Aukerman, the Federal Circuit vacated the district court’s decision and remanded for further proceedings to determine whether “extraordinary circumstances” have been established.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-early-bird-ends-Apr-21-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)







Join the Discussion
One comment so far.
Lost In Norway
May 6, 2018 11:47 amThank you for the great article. I appreciate your keeping me up to date on these cases.