“With this provision struck, the status quo remains, and drug makers will continue to receive only five years of protection in Mexico, and eight years of protection in Canada.”
 President Donald Trump and House Speaker Nancy Pelosi have reportedly reached an agreement on the much debated United States-Mexico-Canada Agreement (USMCA), which would, if ratified, replace the defunct and maligned North American Free Trade Agreement (NAFTA). The agreement, announced by Speaker Pelosi on Tuesday morning, comes as the House prepares to impeach President Trump, which makes it somewhat surreal, given that House Democrats seem poised to deliver President Trump a victory. During his presidential campaign, then-candidate Trump promised at nearly every campaign stop to renegotiate NAFTA.
President Donald Trump and House Speaker Nancy Pelosi have reportedly reached an agreement on the much debated United States-Mexico-Canada Agreement (USMCA), which would, if ratified, replace the defunct and maligned North American Free Trade Agreement (NAFTA). The agreement, announced by Speaker Pelosi on Tuesday morning, comes as the House prepares to impeach President Trump, which makes it somewhat surreal, given that House Democrats seem poised to deliver President Trump a victory. During his presidential campaign, then-candidate Trump promised at nearly every campaign stop to renegotiate NAFTA.
“It is infinitely better than what was initially proposed by the administration,” Pelosi said. “It’s a victory for America’s workers.”
In remarks posted on Twitter, President Trump said: “America’s great USMCA Trade Bill is looking good. It will be the best and most important trade deal ever made by the USA. Good for everybody – Farmers, Manufacturers, Energy, Unions – tremendous support. Importantly, we will finally end our Country’s worst Trade Deal, NAFTA!”
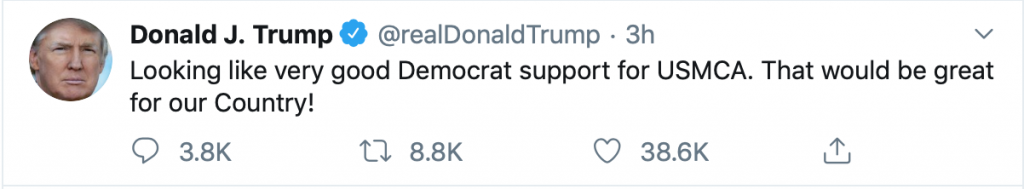 In a second tweet moments later, President Trump said: “Looking like very good Democrat support for USMCA. That would be great for our Country!”
In a second tweet moments later, President Trump said: “Looking like very good Democrat support for USMCA. That would be great for our Country!”
Multiple sources confirm that the latest version of the USMCA agreed upon by the White House and House Democrats strikes expanded protection for biologic drugs from the agreement completely. Over the summer, House Democrats vocally opposed granting 10 years of regulatory data protection (RDP) for biologics inventions—an increase from 8 years in Canada and from 0 in Mexico—arguing it would result in higher drug prices and delayed entry for biosimilars.
In the U.S., biologics currently enjoy 12 years of protection. With this provision struck, the status quo remains, and drug makers will continue to receive only five years of protection in Mexico, and eight years of protection in Canada.
According to CNN, “Democrats opposed enshrining the protections in the agreement because they want Congress to be able to legislate on drug pricing issues without being bound by the trade deal.”
Republicans urged President Trump to keep the 10-year provision for biologic protection in the USMCA, but ultimately, in order to get Democrat support, it was struck. “I think removing it is a better option than weakening those years further,” said Congressman Kevin Brady (R-TX). “At the end of the day, U.S. protections are preserved.”
U.S. Senator John Cornyn (R-TX), declined to endorse the agreement reached between President Trump and Speaker Pelosi. “I look forward to reviewing the agreement, hearing how it will impact Texas and providing feedback,” Cornyn said.
Groups such as the Geneva Network, The Pharmaceutical Research and Manufacturers of America (PhRMA) and the Global Intellectual Property Center (GIPC) at the U.S. Chamber of Commerce have argued that the expansion of the RDP period would have helped to ensure that innovators continue to invest in biologics. Patrick Kilbride of the GIPC wrote on IPWatchdog earlier this year:
Critics claim that RDP will increase the cost of medicines, but research from the Geneva Network illustrates that when Canada and Japan extended the term of RDP in the past, it had no significant impact on pharmaceutical expenditure. In fact, when Canada introduced an eight-year term of RDP in 2006, pharmaceutical expenditure as a percentage of total health spending actually decreased in the years that followed.
In September, following U.S. Trade Representative Robert Lighthizer’s reported willingness to negotiate on the Democrats’ request to reduce the 10-year period of protection, former Department of Health and Human Services (HHS) Secretary Tommy Thompson said that drug pricing debates should be relegated to other avenues, such as Congress and the presidential Administration. “Congress should stop its foot-dragging and vote on the USMCA for what it is: a trade bill that encourages other countries to meet existing U.S. standards,” he said.
Image Source: Deposit Photos
Image ID: 219077908
Copyright: lightsource

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/05/Quartz-IP-May-9-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-last-chance-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
2 comments so far.
Derryll Brudzinski
December 11, 2019 07:01 pmFriends to the north and friends to the south – as it should be .
History will be kind to the Donald
The smoke will have to clear is all .
It will be interesting to see how this will effect IP in other areas .
Luis Figarella
December 11, 2019 10:00 amDo you know if all applications are to be published (as was the goal of the TPP)?