“The CAFC clarified that ‘prior art ranges for solutions of structurally and functionally similar compounds that overlap with a claimed range can [and, in this case, do] establish a prima facie case of obviousness.’”
In Valeant Pharmaceuticals v. Mylan Pharmaceuticals, the Court of Appeals for the Federal Circuit (CAFC) reversed and remanded a district court decision granting summary judgment that a claim of Valeant’s patent directed to an opioid antagonist would not have been obvious in view of similar but different compounds. The CAFC also rejected the district court’s obvious to try analysis and holding.
The Stable Methylnaltrexone Patent
The patent in suit, U.S. Patent No. 8,552,025 (the ’025 patent), was directed to a stable solution of methylnaltrexone- a quaternary amine opioid antagonist derivative for reducing the side effects of opioids. In particular, the asserted claim, i.e. claim 8, depended from claim 1, which recited “[a] stable pharmaceutical preparation comprising a solution of methylnaltrexone or a salt thereof, wherein the preparation comprises a pH between about 3.0 and about 4.0.” Claim 8 added the limitation that the “pharmaceutical preparation…is stable to storage for 24 months at about room temperature.”
Mylan sought approval from the U.S. Food and Drug Administration to market a generic version of Valeant’s drug based on the ‘025 patent, Relistor®. In response, Valeant brought a suit against Mylan in the U.S. District Court for the District of New Jersey, alleging that Mylan’s proposed generic version of the product would infringe the ’025 patent. Conceding that the generic product would infringe claim 8 of the ‘025 patent, Mylan asserted that claim 8 was invalid as obvious over solutions of similar anti-opioids and that the claimed pH range would have been obvious to try.
In particular, Mylan argued that claim 8 would have been obvious in view of references teaching naloxone and naltrexone, which were “structurally and functionally similar to methylnaltrexone.” The structures of methylnaltrexone, naloxone, and naltrexone are as follows:
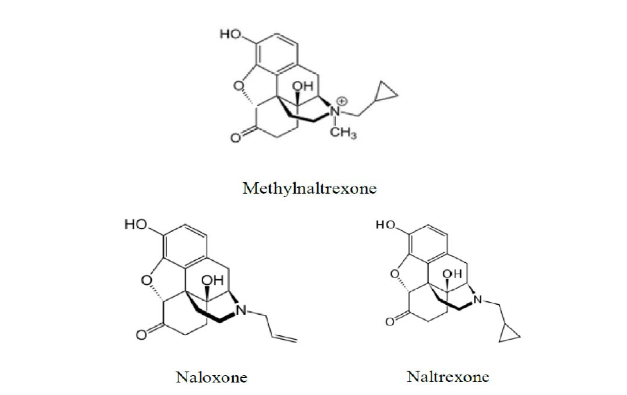
Mylan also argued that the pH recited in claim 8, about 3.0 to about 4.0, would have been obvious to try. The district court disagreed with Mylan because the references did not specifically teach methylnaltrexone. Further, the court noted that “overlapping ranges only establish a prima facie case of obviousness when the only difference between the prior art is the ‘range or value of a particular variable.’” In rejecting Mylan’s argument that the claimed pH range would be obvious to try, the court stated that “given any two unequal numbers, the quantity of number ranges falling between the two is infinite, not finite.” Thus, the district court granted Valeant’s motion for summary judgment that claim 8 would not have been obvious and Mylan appealed to the CAFC.
Obviousness and Obvious to Try Arguments
The Federal Circuit addressed Mylan’s two arguments that the district court erred: “(1) by failing to hold that Mylan established a prima facie case that claim 8 would have been obvious because the pH range in the claim overlaps with pH ranges in the prior art for similar compounds and (2) by resolving disputed fact issues at summary judgment.”
With respect to the first point, the CAFC agreed with Mylan that the cited references established a prima facie case of obviousness because they teach pH ranges that overlap the claimed range. Further, the CAFC agreed with Mylan that even though the references did not specifically teach methylnaltrexone, methylnaltrexone “bears significant structural and functional similarity to both naloxone and naltrexone such that a person of skill in the art would seek to use prior disclosed pHs for naloxone and naltrexone when formulating solutions of methylnaltrexone.” Citing In re Peterson, the CAFC explained that “overlapping ranges [are] sufficient to establish a prima facie case of obviousness, shifting the burden to the patentee to show that the invention would not have been obvious.” In recognizing that none of the cited references specifically disclosed the claimed drug, the CAFC clarified that “prior art ranges for solutions of structurally and functionally similar compounds that overlap with a claimed range can [and, in this case, do] establish a prima facie case of obviousness.” The CAFC also explained that this holding should not be construed to mean that compounds with similar structure and function will “always” be expected to exhibit similar properties. Rather, the CAFC noted, the discussed similarities create a prima facie case of obviousness that may be rebutted by the patentee by showing, for example, that the range is critical, that the differences in the structures provide unexpected results, or by a showing that the prior art teaches away from the claimed invention.
With respect to Mylan’s second argument, the CAFC addressed the district court’s obvious to try analysis and holding that “there was [an infinite, rather than finite] number of options between pH ranges falling between 3 and 7.” Citing KSR Int’l v. Teleflex Inc., the CAFC noted that if there are were a “finite number of identified, predictable solutions” leading to “the anticipated success, the combination was obvious to try.” Contrary to the findings of the district court, the CAFC explained that the bounded range of pH 3-4 presents a finite number of narrower pH ranges to try because even if one number past the decimal point is considered, i.e. 3.0-4.0, the number of pH variables within the range is only ten. The CAFC thus concluded that the district court’s grant of summary judgment for Valeant was in error and reversed and remanded.

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/UnitedLex-May-2-2024-sidebar-700x500-1.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
No comments yet.