“The copious patentable aspects of a therapeutically efficacious siRNA delivery formulation imply intense patenting activity in the years to come, along with market exclusivity that can be extended through strategic patenting activities.”
 Small interfering RNA (siRNA) therapeutics have shown tremendous promise in targeting diseases with poor prognoses, transforming the pharmaceutical landscape. They have allowed a paradigm shift from a conventional inhibitor-based approach to RNA-induced targeted gene silencing. Rational siRNA design and delivery methods have significantly improved their stability, limited immune activation, and increased target affinity resulting in an influx of siRNA-based clinical trials. The U.S. Food and Drug Administration (FDA) has since approved three therapeutic siRNA drugs, ONPATTRO® (patisiran), GIVLAARI® (givosiran), and OXLUMO® (lumasiran) developed and marketed by Alnylam® Pharmaceuticals. In addition, several other drugs are in the late stages of clinical trials.
Small interfering RNA (siRNA) therapeutics have shown tremendous promise in targeting diseases with poor prognoses, transforming the pharmaceutical landscape. They have allowed a paradigm shift from a conventional inhibitor-based approach to RNA-induced targeted gene silencing. Rational siRNA design and delivery methods have significantly improved their stability, limited immune activation, and increased target affinity resulting in an influx of siRNA-based clinical trials. The U.S. Food and Drug Administration (FDA) has since approved three therapeutic siRNA drugs, ONPATTRO® (patisiran), GIVLAARI® (givosiran), and OXLUMO® (lumasiran) developed and marketed by Alnylam® Pharmaceuticals. In addition, several other drugs are in the late stages of clinical trials.
siRNA: An Introduction
siRNAs are naturally occurring, non-coding RNA. The precursors of siRNAs are transcribed by nuclear RNA polymerase II (animals and plants) and nuclear RNA polymerase IV (only in plants). These precursor RNAs are processed by an endoribonuclease ‘DICER’ into siRNA. Typically, siRNAs are 20-25nt in length and are double-stranded. The two strands are the ‘guide strand’ and the ‘passenger strand’. The siRNAs associate with an Argonaute protein to form an RNA-Induced Silencing Complex (RISC). The guide strand of the siRNA directs the RISC to the target messenger RNA (mRNA). The Argonaute cleaves the target mRNA leading to mRNA degradation, preventing any downstream effects. This phenomenon is known as RNAi (RNA interference).
The knowledge gained by understanding the mechanism of action of naturally occurring siRNAs in mammalian cells has opened new possibilities for synthetically designed siRNA as therapeutics. These therapeutic siRNAs can potentially degrade a targeted mRNA involved in a specific ailment, eventually slowing or ceasing the poor disease-related prognosis. The main prerequisites of siRNA designed for therapeutics are a higher degree of siRNA activity and target specificity. Administration of the siRNAs to the target sites is another challenge. The Viral- and lipid-based nanoparticle systems are the most used methods to deliver siRNA.
Patent Scenario of siRNA
Patents have played a crucial role in the emergence and development of RNAi technology. It all started with Andrew Fire and Craig Mello’s groundbreaking discovery of RNA interference (RNAi), protected by the U.S. Patent US6,506,559, which eventually earned the Nobel Prize in 2006. Their patent titled “Genetic inhibition by double-stranded RNA” was assigned to the Carnegie Institute of Washington. The ’559 patent explains a process for introducing a double-stranded RNA into a living cell to inhibit gene expression of a target gene in that cell, i.e., gene silencing by the dsRNA or RNA interference.
The table below summarizes the formative patents that helped shaped the modern RNAi technology landscape.
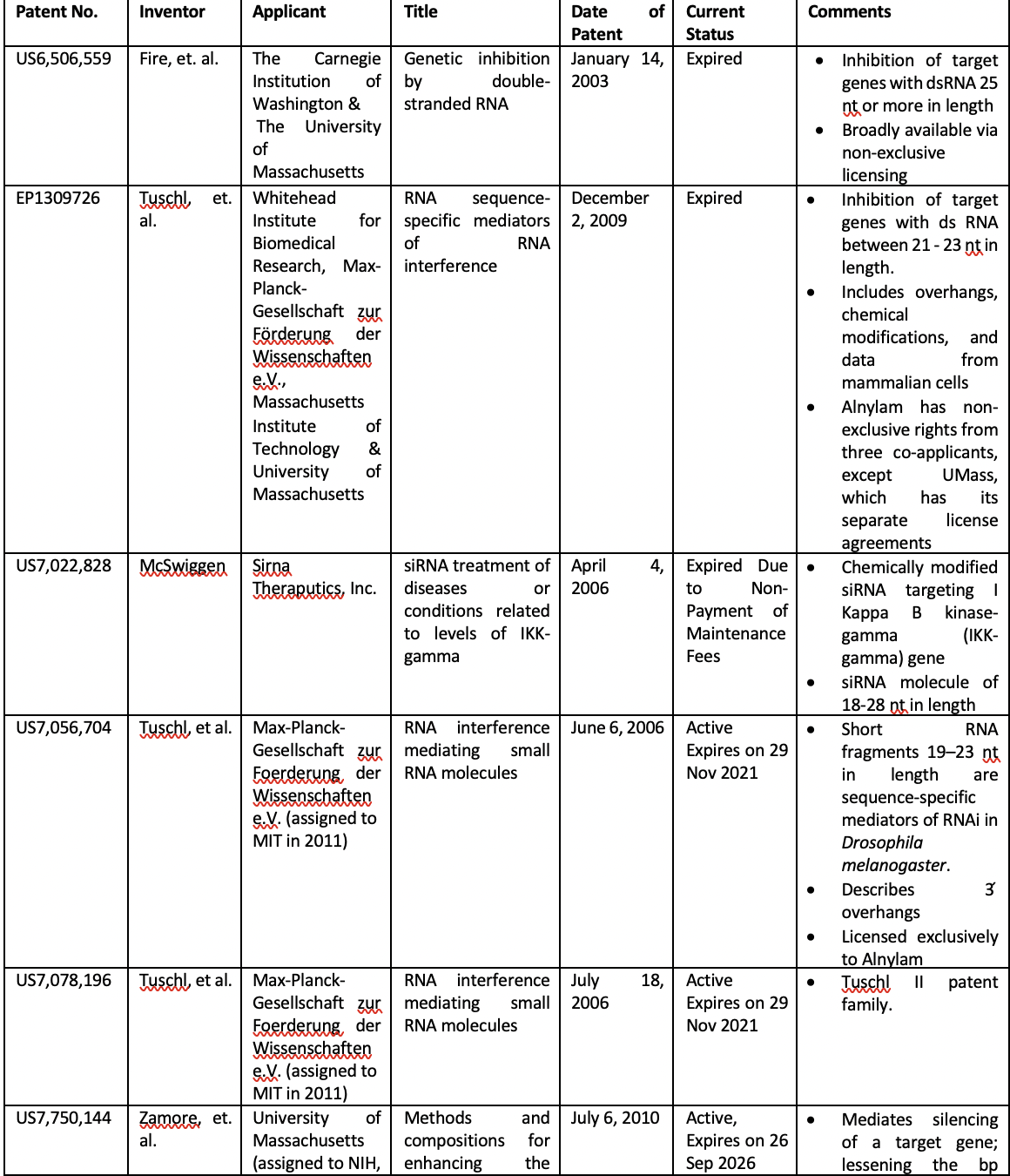
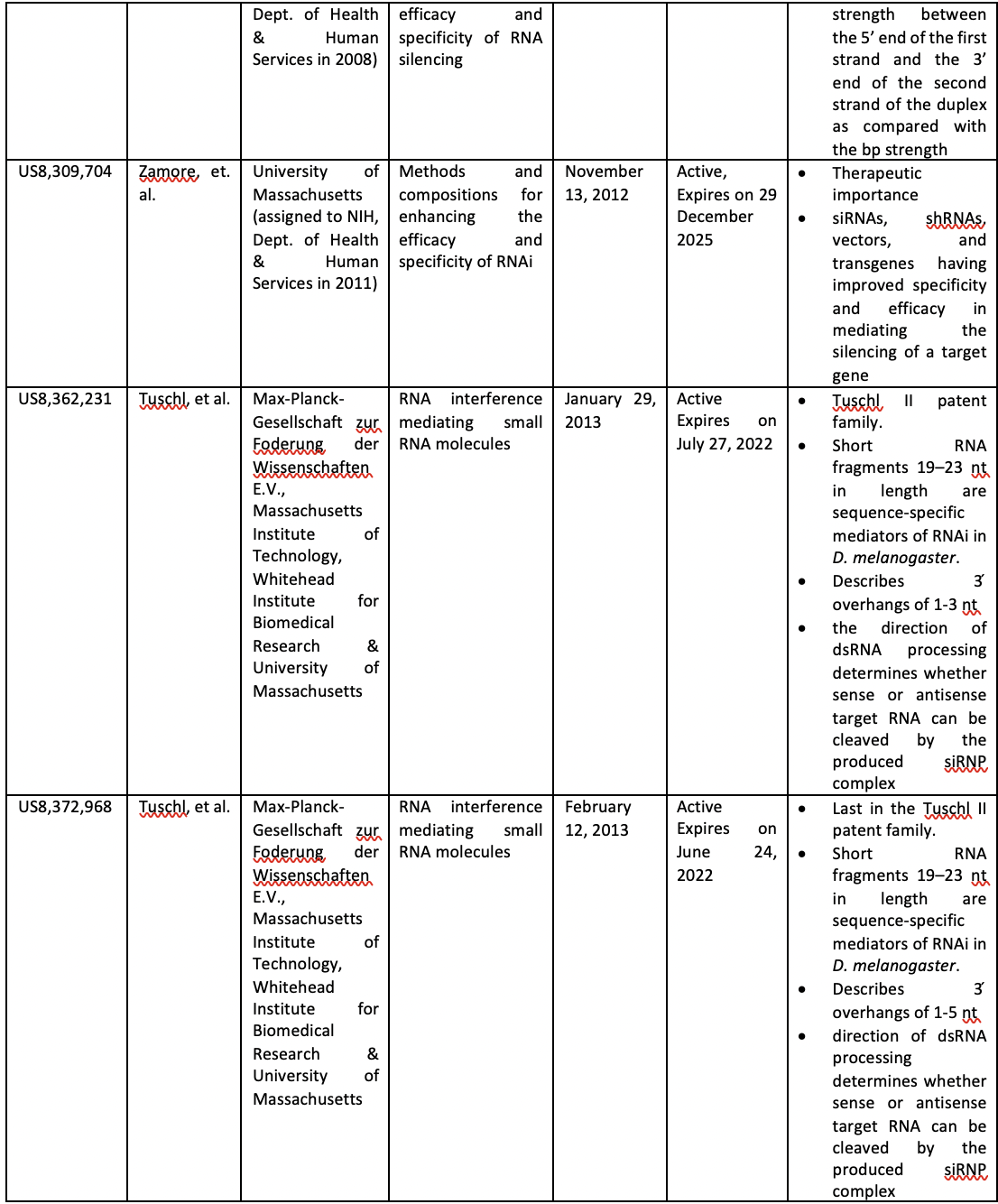
The ‘Tuschl’ patent (family) is often considered as critical patents for making siRNA a therapeutic reality. The Tuschl I patent (EP 1309726) of the patent family titled “RNA sequence-specific mediators of RNA interference” was issued to the Max-Planck Institute, University of Massachusetts, Whitehead Institute for Biomedical Research, and Massachusetts Institute of Technology (MIT). This patent describes the processing of the dsRNA into 21-23 nt RNA segments and their use for precisely inactivating gene function. The Tuschl II patent (EP 1407044) titled “RNA interference mediating small RNA molecules” was granted to the EMBL and Max Planck Institute. This patent describes the 19-23 nt short RNA fragments known as short interfering RNAs (siRNAs) generated by an RNase III-like processing reaction from long dsRNA. Thus, in a nutshell, the Tuschl I patent describes RNAi roughly depicting the siRNA mechanism. In contrast, the Tuschl II patent specifically describes the dsRNA molecules of precise size with the required 3’-overhanging nucleotides. Later this year, the patent term of the Tuschl II family will expire.
The Max Planck Institute exclusively licensed these patented research findings to Alnylam Pharmaceuticals in 2002, making them a leading RNAi therapeutics company. In 2018, this edge earned Alnylam first-ever FDA approval for siRNA therapeutic, ONPATTRO® (patisiran), creating history and ushering into the era of RNAi therapeutics. Patisiran is a siRNA therapeutic for the treatment of polyneuropathy caused by hereditary transthyretin-mediated amyloidosis (hATTR). Twenty-One U.S. patents cover it. In 2019, the FDA approved Alnylam’s GIVLAARI® (givosiran) for acute hepatic porphyria (AHP), also based on Tuschl’s siRNA research. Twelve U.S. patents protect GIVLAARI®. With Oxlumo® (Lumasiran) by Alnylam, a third siRNA drug using Tuschl’s research was approved in 2020 by the authorities in the United States and Europe. Ten U.S. patents protect Oxlumo®. The license revenues for Max Planck Society from the RNAi patents are over $30 million to date. Recently, the FDA approved the IND application for Phase I clinical trial of Sirnaomics, Inc.’s siRNA drug candidate STP707 for treating the solid tumor. Several other siRNA drug candidates are in the late stages of clinical trials. These FDA approvals are enormous milestones for RNAi-based therapeutics.
The early research and rewards created an uptick in the patenting activity of the siRNA in the first half of the decade, followed by the decline. The plausible explanation for this fluctuation in the siRNA’s patenting activity could be that the financial crisis of 2008 might have dried up the revenue stream for this nascent technology leading to the slight fading of the buzz generated during the first half of the decade, thus slowing down the patenting activity. However, the rapid research gains are now compensating for the early setbacks in the siRNA therapeutic field.
Sirna Therapeutics, an Alnylam subsidiary, has got the most patents, followed by the University of California. Interestingly, big pharma is not among the top patent filers; instead, most of them have maintained an indirect presence in RNAi through collaborations, partnered development programs, or strategic partnerships with other giants of the siRNA turf. For example, the top patentee of this domain, Sirna Therapeutics, was acquired by Merck & Co. in 2006 for a whopping $1.1 billion, who then sold it to Alnylam Pharmaceuticals for $175 million in cash and equity.
siRNA Patent Litigation
So far, the two litigations involving ‘Tuschl’ patents – Max-Planck-Gesellschaft v. Whitehead Institute (2009) and the University of Utah v. Max-Planck-Gesellschaft (2011) are the most famous. The Max-Planck-Gesellschaft v. Whitehead Institute patent dispute is also known as the “RNAi litigation,” which involves ownership of the ‘Tuschl’ II patents and violation of the joint agreement between the parties. This litigation was settled in February 2011 before trial on the condition that the Max-Planck-Gesellschaft controls the future prosecution of the ‘Tuschl’ I and ‘Tuschl’ II patent families in the United States. UMass could license Tuschl II to Merck subject to Alnylam’s permission, receiving part of the licensing fees and prosecuting Tuschl II outside the US.
The University of Utah v. Max-Planck-Gesellschaft case was decided in March 2017. It was an ownership dispute involving the Tuschl II patent family and Prof Brenda Bass of the University of Utah. She alleged that she was wrongfully omitted as the inventor of the 3′ overhang feature of the RNA gene silencing mediators in the Drosophila lysate experiments leading to Tuschl II. The University of Utah withdrew their case as they were unable to gather sufficient evidence proving the inventorship of Dr. Bass.
Another patent litigation, Tekmira v. Alnylam (2012), disputed siRNA Lipid Nanoparticle (SLNP) delivery technology. Tekmira alleged that Alnylam violated the patent and licensing agreement and asserted that Alnylam misappropriated confidential information, including trade secrets, by disclosing it to a third party and incorporating it into Alnylam’s patent filings. Tekmira sought an upward of $1billion in damages. The parties settled the dispute, with Alnylam agreeing to pay $65million to Tekmira and restructuring the licensing agreements.
Intense Patenting Activity to Come
Patents combined with trade secrets can pose a significant challenge to anyone designing around the siRNA technologies. The siRNA still has some issues that need to be ironed out to realize its full potential. The siRNA administration and efficient delivery are at the forefront. Efficient delivery methods of siRNA, which are considered the most significant hurdle in the broad launch of the technique, are also the most prominent strength from the patenting and clinical trial point of view. The copious patentable aspects of a therapeutically efficacious siRNA delivery formulation imply intense patenting activity in the years to come, along with market exclusivity that can be extended through strategic patenting activities. The numerous vital features of a therapeutic nanoparticle, such as a siRNA delivery, can confer patentability to a delivery platform targeting moieties that enable reaching specific tissues and cell types. The modified nanoparticle composition, administration modes, dosing regimens, treatment methods, etc., are all open for development and hence probable patentable inventions.
For an industry where it takes at least 15 years to establish a platform, develop drugs, and get them approved, nascent siRNA technology has done reasonably well for itself and has emerged as a new class of therapeutics. Moreover, with the astute use of IP over a short duration, siRNA therapeutics has shown enormous potential for future medical applications, not just for orphan and hereditary diseases.
Image Source: Deposit Photos
Vector ID:89243992
Copyright:logos2012

![[IPWatchdog Logo]](https://ipwatchdog.com/wp-content/themes/IPWatchdog%20-%202023/assets/images/temp/logo-small@2x.png)


![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Artificial-Intelligence-2024-REPLAY-sidebar-700x500-corrected.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2024/04/Patent-Litigation-Masters-2024-sidebar-700x500-1.jpg)

![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/WEBINAR-336-x-280-px.png)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/2021-Patent-Practice-on-Demand-recorded-Feb-2021-336-x-280.jpg)
![[Advertisement]](https://ipwatchdog.com/wp-content/uploads/2021/12/Ad-4-The-Invent-Patent-System™.png)






Join the Discussion
2 comments so far.
George
September 16, 2021 12:40 amInteresting!!! Thanks for the very detailed explanations. Things moving very fast in biology, biochemistry & pharmacology. Who can even keep up anymore? But it’s a fascinating time. Maybe soon we will be able to cure many more diseases. The future of medicine looks much brighter again. Let’s hope.